
Ever send in a part for coating only to find there's a problem with getting it coated? It may not be the coating, it may be the steel. Learn about how sulfide can impact CVD silicon coating quality.
How Sulfide Inclusions in Steel Impact Silicon Coating Quality
SilcoTek coats thousands of parts every day, and while there is a good record of success when attempting to coat substrates that are listed on our compatibility guide, there are always unknowns that can be traced up the supply chain, all the way back to the steel manufacturer. Previously we have seen that surface preparation techniques such as electropolishing, passivation, machining, cleaning, etc. can have dramatic impacts on the application of our coatings. Recently we found that issues can arise from processing steps even further up the supply chain. In this case, we will discuss the impact that manganese sulfide, a common additive in steel, can have on the deposition process, and what can be done to mitigate these issues; and improve the quality of your product and improve performance.
|
In this blog post you will learn:
- How outside factors like manufacturing and cleaning processes can effect CVD coating quality.
- How manganese sulfide can lead to coating quality issues on 316 stainless steel.
- How nanowire growth can impact coating quality.
- How SilcoTek solved a customer material problem and developed a new process method.
|
Have a question about this blog or material properties? Click the box below to discuss your application with one of our coating scientists.
Have a Question? Contact Our Technical Service Team
Background:
SilcoTek Corporation has a long history of performing chemical vapor deposition on a variety of metallic and non-metallic substrates. Occasionally, SilcoTek encounters issues with coating a specific part even though the substrate is on the coating compatibility list (a brief summary of what substrates can and cannot be coated can be found here). In one example, a machine shop that was manufacturing stainless steel components also machined brass components. Shared tooling between the substrates caused copper deposits on the surface of the stainless steel which caused abnormal nanowire growth. In another example a customer's cleaning method upstream from SilcoTek had a dramatic impacts on the coating quality. Recently, it has been shown that metallurgical issues in raw materials can cause coating defects, even when the raw material is a compatible alloy for our process.
This blog will discuss a recent investigation that led to a discovery that high amounts of manganese sulfide in 316 stainless steel can lead to coating issues. Details regarding the coating defect and solutions for properly coating the troublesome parts will be discussed. Have trouble solving a difficult material challenge? Contact our Technical Service Team, or subscribe to our coating blog.

Problem and Analysis:
SilcoTek Corporation recently had an issue with a customer part that resulted in poor coating quality. An investigation was launched to assign the cause of this abnormal coating quality issue. The part was cylindrical in nature and the surface on the two faces of the cylinder had abnormal growth that appeared dark and splotchy as seen in Figure 1. The outer surface of the cylinder looked pristine.

Figure 1: Top surface of the cylinder-shaped part. The dirty marks are abnormal growth which is not typically found in our process.
As shown in Figure 2, SEM analysis revealed that the growth consists of extremely long nanowires. EDS showed that these nanowires were made of silicon, and no other abnormal elements were present in the nanowire bundle. Macroscopically, the areas where the nanowires are present appear to be dark due to the scattering of light when hitting the mass of nanowires. This light scattering is a similar mechanism that ultra-black material uses to absorb light.

Figure 2: Scanning electron micrographs of the nanowire bundles that cause the surface to look black.
Nanowire growth during high temperature silicon deposition can be caused by a large number of contaminants on the surface of stainless steels. The cause of such growth is typically a vapor-liquid-solid mechanism that is well documented in literature. Gold is usually used for this type of growth; however, there are many elements and alloys that can cause this growth. One additional clue to solving the puzzle came when the parts were immersed in nitric acid. A 10% nitric acid bath was prepared, and the parts were soaked for 30 minutes at room temperature. The parts became hazy on the top and bottom surface after the exposure. Typical stainless steel would be unaffected by exposure to nitric acid at this concentration and temperature as seen here. The cause of the haze was microscopic pits that developed on the surface as seen in Figure 3. Upon discussing these issues with the customer, it was discovered that during the summer of 2018, they changed steel suppliers, which naturally lead to the question: what is different between these two steels?

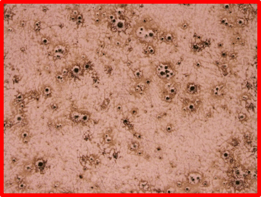
Figure 3: Surface of troublesome part after nitric acid bath soak. Left is a photo of the hazy looking part. Typically, this part has a high polish on this surface, but exposure to nitric acid caused a hazy appearance. The optical micrograph on the right shows pitting on the surface of the part causing effect. The pitting indicates that there is an impurity on the surface which may be causing the nanowire growth.
Steel Quality Variation
Usually the various elements added to make a specific steel alloy have an acceptable concentration range for each element or are given a maximum allowable concentration. This leads to slight variability from lot to lot of steel. Additionally, there are certain steels that are sold as “free machining” steels. These steels often have higher concentrations of sulfur than their cleaner counterparts. This addition will form manganese rich sulfides which slightly soften the steel allowing a cutting tool to operate at higher speeds and have extended lifetimes. There are also disadvantages to using such steels. They often experience inferior corrosion behavior (both pitting/crevice corrosion and stress corrosion cracking), lower ductility and toughness, and as shown here, they can cause abnormal growth in CVD coating processes. The machine shop that produces these parts changed to a high sulfur steel in the summer of 2018. After this discovery, and the investigations that occurred at SilcoTek®, the machine shop switched their raw material back to the original “clean” steel and the first run pass rate went back to near 100% for our coating process. Have a material compatibility question. Read more about compatibility and CVD silicon coatings.

There was an additional confusion as to why the abnormal growth only occurred on the top and bottom of the cylindrical shaped part while the external surface appeared to be unaffected. The explanation has to do with the processing of the raw material prior to the machining process. The stainless steel is typically mixed in the molten form and then cast into a billet (a rectangular bar). This casting process includes various heat treatments to insure proper chemical and physical properties. From there, the billet is extruded into a cylindrical bar stock as shown in Figure 4A. Any manganese sulfide inclusions in the billet are typically round in nature and evenly dispersed through the stainless steel. When the extrusion process occurs, it forces those inclusions to elongate in the axial direction. These elongated inclusions are commonly known as “stringers”. Since the stringers all run in the same directions, when the part is machined, the top and bottom will have numerous exposed manganese sulfide inclusions whereas the outer surface will have far less as shown in Figure 4B.
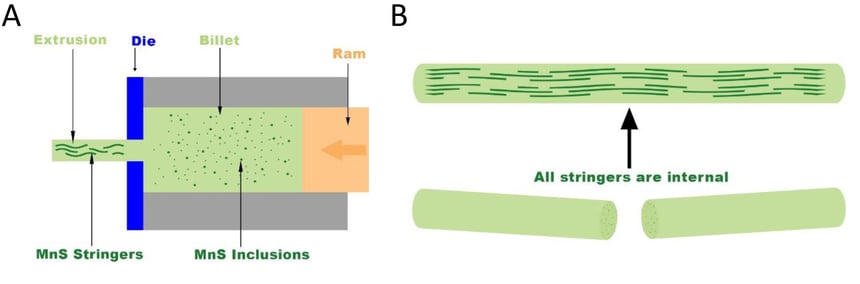
Figure 4: A shows the extrusion process forming the rounded bar stock. It takes evenly distributed manganese sulfide inclusions which are round in shape and elongates them along the axial direction. B shows the stringers running along the length of the bar stock. When cut, the manganese sulfide inclusions are concentrated on the cut section which explains why the abnormal growth only occurs on the top and the bottom of the cylindrical part.
Experimental
To confirm that manganese sulfide leads to abnormal growth, two 316L stainless steel QC coupons were scratched and then one of them was rubbed with manganese sulfide powder which was purchased from Sigma Aldrich. The coupons were then blown off with nitrogen to remove loose manganese sulfide and retain only the MnS powder that was trapped in the scratched trenches. The two coupons were then coated with SilcoNert® 2000. As seen in Figure 5, the coupon that was not rubbed with manganese sulfide only showed scratches whereas the coupon with manganese sulfide exhibited short silicon whisker growth. It should be noted that manganese sulfide inclusions in steel are compositionally different than pure MnS, so the growth morphology is not identical to what we saw on customer parts.
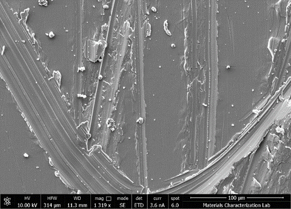

Figure 5: Two coupon were scratched and one was rubbed with manganese sulfide powder (right) prior to coating with SilcoNert 2000. The coupon without the powder appeared normal, and SEM showed only the scratches, where the coupon rubbed with manganese sulfide powder has islands of short whiskers.
Solution for Improved Process Reliability
Lastly, SilcoTek Corporation cannot control the grade of steel that customers choose to use when machining their parts. Should a part have high amounts of manganese sulfide inclusions at the surface, a standard SilcoNert deposition will result in nanowire growth and a poor appearance. Experimental recipes for depositing SilcoNert 2000 were performed on the customer parts that were known to have high concentrations of the inclusions. The first alternative recipe is one that was developed specifically for aluminum processing. The second is a recipe that was developed to tackle brass/copper contamination on parts that might have shared tooling. The result? Improved process control. A future blog post will discuss the details of these recipes. Figure 6 shows a part that has high concentrations of manganese sulfide and is coated with a new specialized process recipe optimized for management of nanowire growth.

Figure 6: Image of a part coated using a specialized recipe designed to suppress abnormal nanowire growth.
Conclusion:
SilcoTek corporation has a list of substrates that can be successfully coated with SilcoNert 2000 based on past experiences; however, not all alloys are made identically. Some stainless steel alloys specifically include high amounts of sulfur to make them easier to machine. Higher amounts of sulfur in stainless steel will result in higher numbers of manganese sulfide inclusions. These manganese sulfide inclusions can cause issues in our coating process. More specifically, they will cause nanowire growth rather than smooth film growth, resulting in unacceptable cosmetic blemishes. Mitigation of the nanowire growth can be done in two ways. First, a higher quality of steel can be used. This will eliminate the problem of the high number of inclusions and thus eliminate the wire growth. Alternatively, SilcoTek Corporation has developed specialty recipes that are successful in stopping the wire growth from occurring when such inclusions are present, despite the presence of undesirable inclusions.
Have a question about your materials or how CVD coatings can improve your process and products? Ask the experts or join us on LinkedIn.












