Chromatographers use Bio-inert HPLC systems to prevent interaction with stainless steel surfaces. They may not realize that bio-inert doesn't mean metal free. Keywords: Bio-inert, metal free, HPLC, metal ion leaching, inertness
Identifying and Preventing Metal Ion Leaching in HPLC Systems
Many liquid chromatographers move toward bio-inert systems to protect their molecules/proteins from having negative interactions with stainless steel surfaces in normal HPLC equipment. What they may not realize is that just because it’s bio-inert, it doesn’t mean that it’s metal free and if not run properly, titanium ions can easily flood the system and cause issues with any metal active compound. This blog focuses on a paper written by scientists at Thermo Fisher Scientific. They found that pure organic solvent can potentially lead to separation issues that may not be anticipated by most chromatographers.

|
In this blog post you will learn:
- How titanium metal ion contamination can lead to separation issues in HPLC systems.
- How organic solvents can leach metal ions from titanium.
- How inert CVD coatings can prevent metal ion contamination.
Keywords: Bio-inert, metal free, HPLC, metal ion leaching, inertness
|
Background:
A previous blog focused on the rise of biologics and biosimilar pharmaceuticals in the past decade, and how Dursan can lead to better analysis when separating multisialylated glycans. Coinciding with this rise of the biopharmaceutical market is the rise of HPLC manufactures making biospecific systems. Stainless steel is not considered to be a bio-inert or bio-compatible material. Many proteins and biological molecules will interact and/or stick to the stainless steel surface. Alternative materials such as PEEK, titanium, Hastelloy, and MP35N are often used in place of stainless steel as they are considered “bio-inert”. When considering price and strength, titanium is often the material of choice for parts exposed to high pressures such as pumps, mixers, and columns.
Titanium is corrosion resistant to phosphate and MES buffers with high salt concentrations, which makes it an even more attractive material as these solvents are often used when analyzing proteins. A potential issue with using titanium is that it is susceptible to corrosion when exposed to pure organic solvents. This is something that is well known and understood in the material science world, but not well known throughout the chromatography community. While this corrosion is not typically enough to cause mechanical failures, it could potentially have an impact on the chromatography. Laboratories and QA/QC groups that focus on biologically related materials may believe that purchasing a bio-HPLC system will ensure good chromatography, but metal contamination of the system can lead to unexpected consequences.
This blog will summarize a study done by Thermo Fisher Scientific that was recently published in the Journal of Chromatography A, where they investigated the corrosive behavior of pure organic solvents on titanium HPLC components and the effects it has on certain metal active compounds. Learn about how SilcoTek coatings can make HPLC flow paths metal free and bio-inert.

Data and Discussion: Using ICP-MS to Trace the Source of Metal Ion Contamination
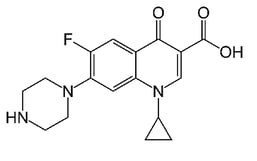
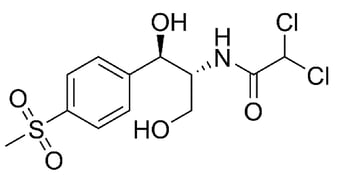
The most relevant study in this paper focuses on two molecules: ciprofloxacin and thiamphenicol. Both are antibiotic compounds that can be utilized to treat various bacterial infections in humans and animals. The molecular structures are shown in Figure 1. Ciprofloxacin contains a carboxylic acid and ketone functional group separated by one carbon. This combination can chelate with metals and metal ions.
Figure 1: Molecular structure of ciprofloxacin (left) and thiamphenicol (right). A portion of ciprofloxacin can chelate to metal ions. *
In their study, they saw that an iron-free, bio-HPLC system can have significant peak tailing if the method utilizes a pure organic solvent. In this case they use a 50:50 mixture of methanol and acetonitrile with 0.05% formic acid. To identify the source of the peak tailing, they systematically replaced every part of a prototype instrument that did not show peak tailing with components from a system that was experiencing tailing. The authors did not share any clues as to why the prototype system was not experiencing peak tailing, but they mentioned it is currently under investigation. The results from the systematic replacement can be seen in Figure 2. They found that when a component was replaced on the prototype system with a part that was made of titanium (highlighted in red), there was a dramatic increase in the asymmetry of the ciprofloxacin peak. The thiamphenicol peak was constant throughout the testing.
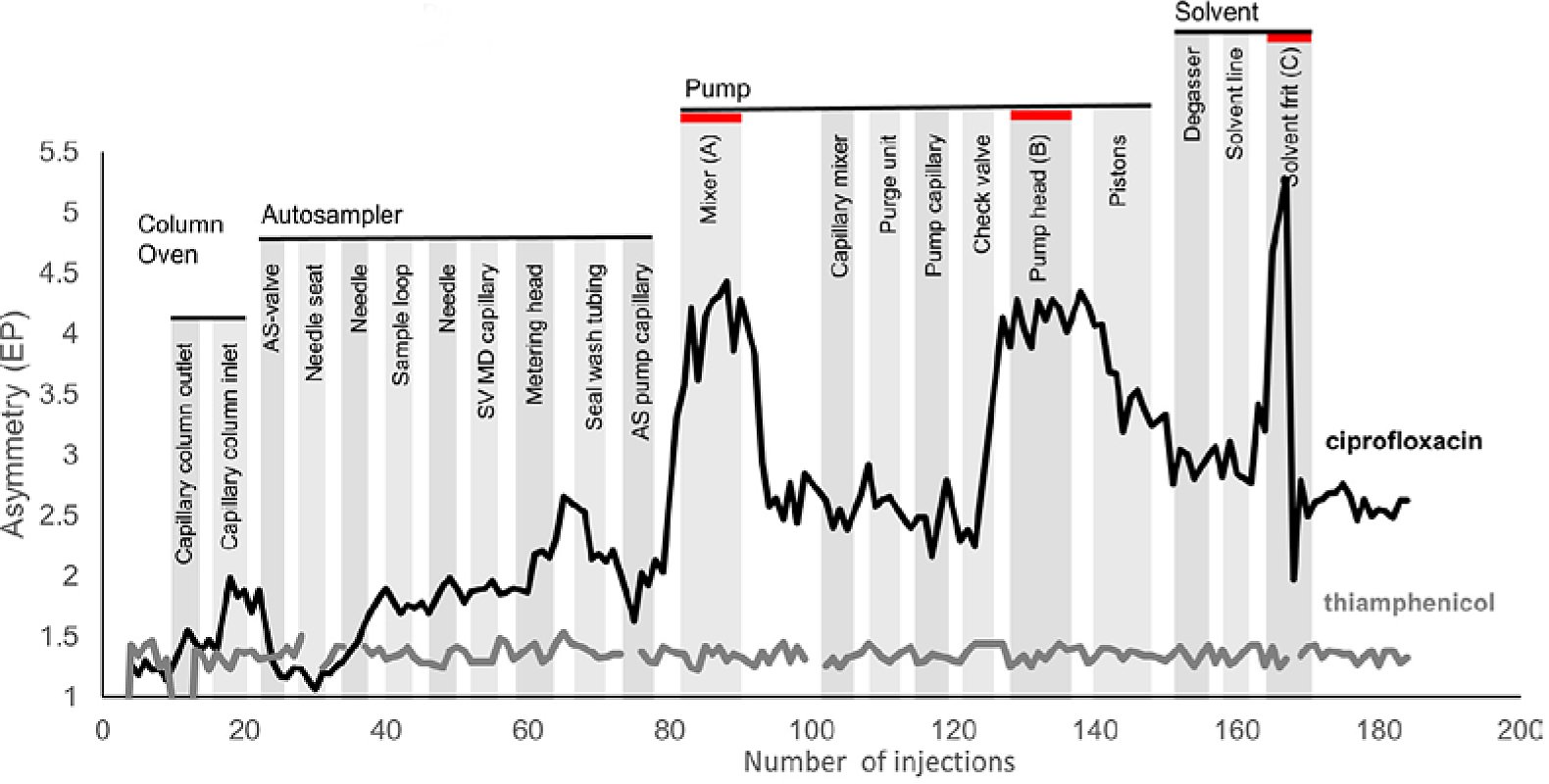
Figure 2: Asymmetry of the ciprofloxacin and thiamphenicol peaks during a systematic replacement of components of the prototype system that was not experiencing peak tailing. *
The authors noted two points of interest in their analysis. First, the components which were responsible for the most peak tailing are those that are not in contact with the analyte. They are all upstream from the injection, so any direct interaction between the parts and the sample can be ignored. Secondly, when moving on to the next component for testing and replacing the prototype part, the asymmetry starts to recover after a few injections, but never back to baseline levels. They suspected that titanium was leaching into the system and to verify this, the group removed the column and tested the solvent pumped through the prototype system with and without a titanium solvent frit via ICP-MS. They found that without a titanium solvent frit, the titanium levels in the effluent were 0.24 ppb. When a solvent frit was used, the titanium levels jumped to 2.13 ppb.
A common method for removing metal ion contamination from an HPLC system is to flush it with EDTA. EDTA has the ability to strongly bind to most metal ions and remove them from the system and column. It was noted by the authors that this technique did not cause full recovery to baseline levels of asymmetry, suggesting that the titanium ions are introduced into the system and become immobile. In other words, once the titanium ions hit the silica bed in the column, the column is permanently contaminated with titanium and any molecule in the sample matrix that is prone to chelation will have poor chromatographic peak shapes and recovery.

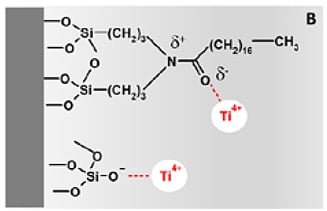
The authors performed numerous experiments to verify that the titanium was bound to the silica in the column of the HPLC system. They found that the silica chemistry is the most important factor that determines the extent of titanium immobilization. When using Thermo Fisher’s Acclaim PolarAdvantage II column, they saw a much higher amount of asymmetry in the ciprofloxacin peak than when using a conventional C18 column. The Acclaim PolarAdvantage column can separate polar and non-polar compounds simultaneously which can be important to the pharmaceutical, food and beverage, environmental, and life sciences industries. This is accomplished by incorporating polar groups into the bonding chemistry as shown in Figure 3. These polar groups and residual silanol groups on the surface of the silica can act as binding sites for the titanium ions. In a conventional C18 column, only the surface silanols are capable of immobilizing titanium ions. It should be noted that column manufacturers optimize their bonding chemistry to eliminate as many silanols as possible. One technique that is often utilized is endcapping the silica with TMS to eliminate the chemically active silanols. A well bonded C18 column may not contain enough silanol groups to show a high amount of bonded metal ions, but any bonding chemistry that does not utilize endcapping or contains polar groups in the bonding chemistry such as cyano, HILIC, or zwitterionic columns are susceptible to high amounts of metal contamination.
 |
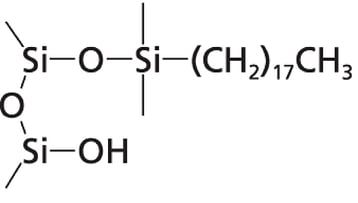 |
Figure 3: Surfaces of silica packing material. On the left is the surface of a Thermo Fisher Acclaim PolarAdvantage II silica particle. The polar groups that are incorporated into the bonding chemistry can bind with titanium ions. A traditional C18 column (right) only has residual silanol groups capable of bonding to metal ions.
An interesting solution that the authors found was that incorporating a small amount of water (5% by volume) into the organic solvent container, they can almost eliminate the titanium leaching problem. This is a simple solution, but it could cause issues with validated methods where a change in the procedure is non-trivial. Additionally, this is a relatively unknown phenomenon among chromatographers. A more universal way to eliminate the leaching problem would be to utilize SilcoTek Corporation’s coating technology. SilcoTek coatings, like Dursan, act as an inert, non reactive barrier, preventing interaction of the analyte with the flow path substrate material (be it titanium or stainless steel).
The corrosion of titanium in the presence of neat methanol has been known since the 60’s when NASA found that Ti-6Al-4V (a common titanium alloy) will experience stress-corrosion cracking when exposed to methanol. Since titanium is the metal of choice for many biocompatible HPLC systems, it's critical to eliminate the exposure of the metal components to pure organic solvents. Mauro De Pra of ThermoFisher Scientific presented a talk on this subject at HPLC 2019 in Milan. He showed that titanium ions will migrate from the solvent frits to the silica packing material in the HPLC column. Once there, the titanium ions will react and bind with silanols on the surface of the silica. These immobile ions can cause issues with certain compounds which chelate to them. One such compound is ciprofloxacin, a common antibiotic, which will show significant tailing and peak distortion if run through a titanium contaminated column.
This issue is not limited to titanium. Mel Euerby is currently a scientist with Shimadzu, and he wrote a paper in 1995 while he worked for Fisons Pharmaceuticals (now a part of AstraZeneca) that showed a similar issue can occur with stainless steel. He showed that both acetonitrile and methanol could cause iron ions to leach into the silica bed causing poor peak shapes when running a separation with a metal chelating compound, 2,3-dihydroxynapthalene. He also showed that soaking frits in methanol can extract up to 1µg of total iron, which is not measurable via visual changes nor via mass change with a standard lab balance.
SilcoTek has previously shown that the Dursan coating process can assist with any metal chelating compound separation by providing a barrier layer between the stainless steel substrate and the analytical flow path. This blog post will investigate whether Dursan and other SilcoTek coatings can prevent metal ions from leaching into pure organic solvents.
Why Use Dursan CVD Coating Technology in HPLC
Recent testing demonstrates the effectiveness of silicon barrier coatings in preventing ion leaching and contamination. In the test porous metal discs with 10 µm nominal pore sizes were purchased from McMaster Carr. Coatings to be tested were Dursan, and Silcolloy. Once the coatings were complete, the coated frits and an uncoated frit were placed individually in polypropylene plastic containers which were filled with 100 mL of HPLC grade methanol and closed. One plastic container was filled with 100 mL of HPLC grade methanol and closed without a sintered disc present to act as a baseline for the experiment. After an exposure time of one month, the sintered discs were removed from the methanol and the samples were delivered to the Energy and Environmental Sustainability Laboratories at Penn State University. Samples were then prepared by evaporating 10 mL of the methanol in a PTFA vial. The remains were dissolved in approximately 5 mL of dilute nitric acid which was then analyzed using a Thermo Fisher iCap RQ ICP-MS. Iron, nickel, and chromium were measured and the results can be found in Table 1.
Table 1: ICP-MS results of sintered discs soaked in methanol for one month. All values are in PPB.
|
|
Iron
|
Chromium
|
Nickel
|
|
Blank (no disc)
|
8.22
|
0.11
|
0.26
|
|
Bare disc
|
85.7
|
3.44
|
11.0
|
|
Dursan
|
2.34
|
0.14
|
0.23
|
|
Silcolloy
|
1.55
|
0.07
|
0.22
|
The uncoated sintered disc showed a significant increase in the amount of metal ions that have leached into the solution. It should be noted that the metal content in the Dursan and Silcolloy coated discs appear to be lower than the blank. These values may seem odd since it appears that Dursan and Silcolloy have an adsorptive property to them when it comes to some metal ions. Nothing in our previous testing indicates this to be true.
Learn more about SilcoTek's coating technology.

Conclusion:
Many separation scientists utilize bio-HPLC instruments to ensure that the analytical flow path does not contain any stainless steel as iron can cause issues while separating certain molecules (whether they be small molecules or macromolecules like proteins). They often do not consider the replacement material, such as titanium, and how it can have an effect on the chromatography. A group from Thermo Fisher found that using pure organic solvent can leach titanium ions from numerous sources upstream from the injection site. These ions become immobile in the silica bed of the column, and if the molecule of interest has any chelating ability, the separation can experience peak tailing and poor recovery. Solutions to this issue include incorporating a small amount of water into the organic solvent container or utilizing SilcoTek Corporation’s coating technology to block the ions from leaching into the system.
Have questions about our coatings and how they perform in your application? Ask the experts! Or join the conversion on LinkedIn.

* Images courtesy of: "Effects of Titanium Contamination Caused by Iron-Free High-Performance Liquid Chromatography Systems on Peak Shape and Retention of Drugs with Chelating Properties"; Mauro De Pra, Giorgia Greco, Matthias P. Krajewski, MarkusM. Martin, Ed George , Nora Bartsch, Frank Steiner