
by Dr. David Smith
Surface hydrophobicity & smoothness have a very large effect on contact angle values on glass and metal surfaces. Below is a comparison of contact angles with chemically identical surfaces, but with rough or smooth topographies on 316 stainless steel coupons:

DI water on treated surfaces: Rough

DI water on treated surfaces: Smooth
So with glass vials, we are obviously dealing with a smooth glass surface.

A smooth surface has one strike against it because smooth surfaces produce lower contact angles, but there is more. A “rough” surface may show an artificially high contact angle. However; the rough surface may effect the “mobility” of that droplet and may or may not allow droplet movement in a desired or predictable way (i.e. like a ball bearing may move around on a tilted surface).
There are two types of interactions between a liquid drop and the rough surface it is in contact with.
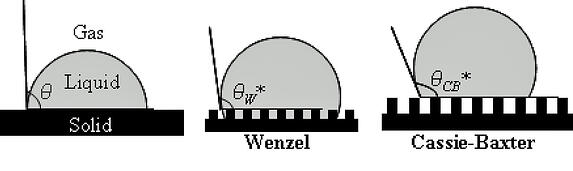
Source: Microstruct superhydrophobic.png. In Wikipedia. Retrieved August 23, 2012, from http://en.wikipedia.org/wiki/File:Microstruct_superhydrophobic.png
The Cassie-Baxter state requires not just surface roughness, but a heterogeneous surface roughness. Since there is essentially zero roughness on a glass surface, the conformal Dursan deposition (i.e. it does not create roughness to the surface) will not allow a classic Cassie-Baxter state. Once the contact angle on a smooth surface approaches approx. 120°, the angle is extreme enough that one will start to see the ball-bearing effect. Also, higher volume drops of contact angles > 90° have enough mass to fall away or roll off a surface. Lower volume drops that do not have enough mass, and are therefore exhibiting Wenzel, non-mobility behavior.
Conclusion:
In a Wenzel state, the drop may have a high contact angle, but there is wetting of the drop down through the surface roughness. This wetting acts as an anchor and prevents mobility of the drop. In a Cassie-Baxter state, there is no wetting of the roughness structure, and the air gap throughout that substructure translates to no “anchor”, and frees the drop to move around (like a ball bearing).
See a drop bounce like a ball bearing!
Learn more about hydrophobicity and how to manage effects of moisture.





