 For maximum reliability, analytical systems used in sampling and transfer of sulfur containing species, system inertness must be addressed when stainless steel components are used. In most refining and petrochemical streams, analysis in the ppm level is required.
For maximum reliability, analytical systems used in sampling and transfer of sulfur containing species, system inertness must be addressed when stainless steel components are used. In most refining and petrochemical streams, analysis in the ppm level is required.
Figure 1, demonstrates the need for coating during the sampling, storage and analysis of part-per-billion levels hydrogen sulfide. In critical applications, the ultimate inertness of components is enhanced using silicon based coatings at the cost of physical durability. In Figure 1 and 2, the degradation of hydrogen sulfide on bare stainless steel is rapid and irreversible: Both at 50ppm and 17bbp levels, H2S is lost within 24 hours.
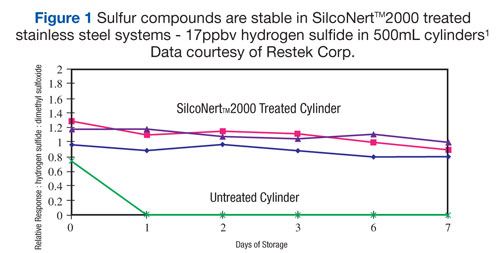
Figure 2, demonstrates that even at concentrations of 50ppm, hydrogen sulfide sampling requires passive surfaces. In this analysis, sample cylinders tested were either sourced from the manufacturer, non-coated, or treated with a carboxysilane, commercial name Dursan™.
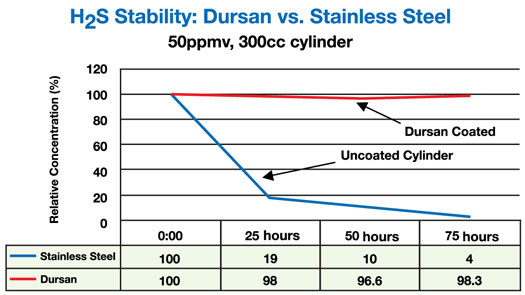
Figure 2: Sulfur Compounds at 50 parts-per-million in
carboxysilane treated Stainless Steel Containers versus non-treated cylinder
The effect of passivation on sulfur storage and transport reliability is debated. Passivation is a technique that is based on the assumption that if all active areas of a transport vessel or storage vessel are taken up by sulfur compounds, they are made inert to sulfur compounds. There have been studies to support this at low temperature for gas phase transport through low surface area regulators. It was demonstrated that purging a component with clean gas can reduce the inertness of the passivation with measurable impact occurring within 1 day and complete within 1 week. Additional data in the same study also demonstrate that heated stainless does not passivate and complete adsorption of sulfurs will occur no matter the conditions and previous exposure to sulfur compounds.
In work to test the stability of sulfur compounds during static sampling, as in sample cylinders, the use of gases such as silane (SiH4) along with multiple day exposure to 5000ppm H2S was required to create a passive cylinder for storage. Much of the data in these studies was done to demonstrate stability for the use of creating low-level standards.
Commercially available inert coated components have eliminated the need for passivation and are now recognized as a “use out of the box” solution to sulfur sampling and transport reliability. This eliminates the need for working with dangerous materials such as high concentration H2S or pyrophoric gases such as silane. The value delivered by coating solutions cannot be taken for granted in comparison to passivation techniques which increase the risk of obtaining poor analytical results.
 For maximum reliability, analytical systems used in sampling and transfer of sulfur containing species, system inertness must be addressed when stainless steel components are used. In most refining and petrochemical streams, analysis in the ppm level is required.
For maximum reliability, analytical systems used in sampling and transfer of sulfur containing species, system inertness must be addressed when stainless steel components are used. In most refining and petrochemical streams, analysis in the ppm level is required. 

