Fouling in HPLC flow paths can lead to frequent maintenance, lost productivity and poor chromatography; and that's the best case scenario! If only there was a way to reduce fouling and improve system performance. You guessed it, there is!
Identifying Fouling in HPLC Systems
Fouling can be caused by high mobile phase viscosity, non specific protein binding, pump problems, flow path corrosion, or voids / crevices in the flow path. The signs of fouling problems include:
- Spike in the pressure trace during injection
- Low flow
- Ghost peaks from carryover or poor peak shape
- Frequent plugging of system
- Column damage
The cause?
Causes of fouling can be as straightforward as a high viscosity analyte but can also be less obvious. Corrosive mobile phases can pit flow path surfaces, making great hiding places for sticky molecules like proteins. System material selection may result in analytes binding to the surface and fouling the flow path. Even sonication during maintenance cleaning can result in surface pitting over time, opening the opportunity for sample buildup in the cavity and carryover..
Prevent Fouling
To manage fouling you need to take a multi faceted approach to the problem. Keys for managing build-up include:
- Understand how materials perform in the application
- Improve corrosion resistance
- Prevent sticky compounds from binding to the surface
- Understand sonication effects on materials
Fortunately there are solutions to fouling and corrosion problems that can be easily implemented. And you guessed it, involve inert silicon coatings!

Material performance in HPLC flow paths
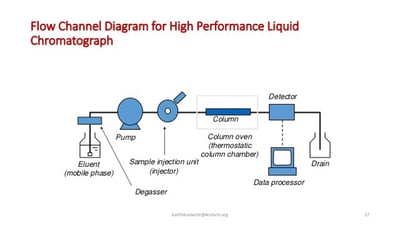
Common sample flow path materials like stainless steel and PEEK can handle many LC applications but the most challenging applications can expose weaknesses in the flow path.
|
Stainless Steel Issues
Acid Corrosion (Halogenated Solvents - HCl, HBr)
Ion Chromatography
Anionic Compounds (phosphates may chelate)
|
Peek Issues
Temperature limitations(Tg148°C)
Halogenated solvent damage
Tetrahydrofuran
|

Preventing Carryover and Protein Binding With Anti-fouling Coatings
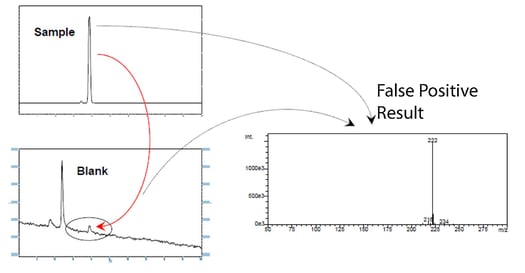
What is sample carryover?
Carryover is the result of a sample binding to the flow path surface which then releases or elutes during a later sampling event. The result is that peaks from the previous analysis may appear is a later test results. The carryover contamination may corrupt later tests, causing false positive results.
Dursan® is a metal free coating that prevents protein carryover and fouling which can lead to carryover. Because proteins don't stick to Dursan, rinse and sonication becomes much more effective. Read the complete study.
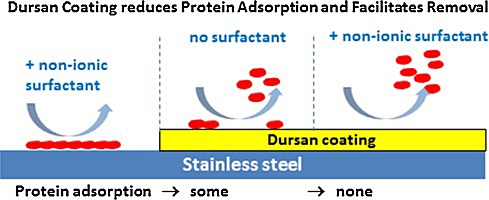
Experimental data show Dursan significantly reduces protein build-up and carry over in HPLC flow paths.
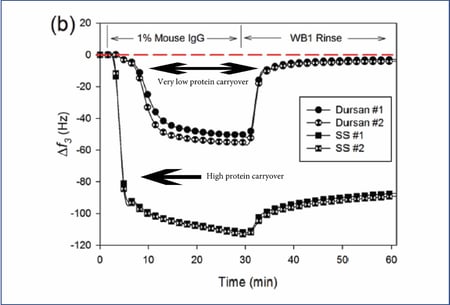

Effect of sonication on coating performance.
LC flow path components are often sonicated to remove contaminants from the surface. Unfortunately protein resistant coatings like AF1600 can be damaged by sonication. Wear resistance tests using sonication demonstrates the robustness of the CVD applied Dursan coating. In contrast AF1600 coating lost efficiency due to coating delamination
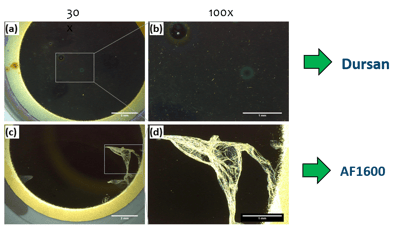
Optical micrograph of the post-sonication QCM-D sensors showed intact Dursan
coating (a and b), compared to delaminated AF1600 coating (c and d).
Corrosion and Ion Contamination
Chlorides (like HCl) and bleach can corrode stainless steel flow paths, causing ion contamination and pitting of the LC flow path. A metal free corrosion resistant coating like Dursan prevents contamination of the test sample.

The test below shows the extent of contamination that can occur as a result of exposure to corrosive analytes. Test coupon immersion in hydrochloric acid solution demonstrates how badly contaminated a solution can become. After only a brief exposure to HCl, the uncoated stainless steel coupon solution is contaminated.
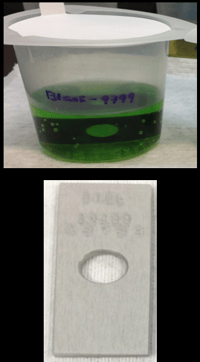
The Dursan® coated coupon solution shows no contamination, assuring a repeatable high quality test.
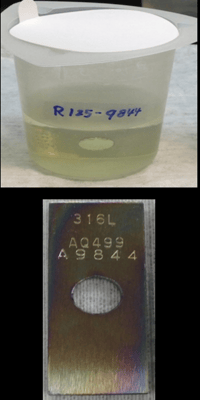
The need for a durable metal free sample pathway.
An inert, corrosion resistant, metal free HPLC coating such as Dursan® solves sampling flow path issues:
Dursan Solutions
- Anti-Corrosion, especially HCl and bleach attack.
- Resistant to saltwater corrosion
|
 |
Want to learn more about LC and GC test inertness and durability? Read our LC-GC presentation.

*Image courtesy of Abbott Laboratories













