 Hydrogen fueled vehicles are popping up around the country. Vehicle manufacturers are offering hydrogen power as a way to achieve low carbon emissions. Unfortunately hydrogen fuel cells are susceptible to damage from impurities commonly found in hydrogen. H2S, ammonia, and carbon monoxide can dramatically shorten the life of fuel cell catalysts. In order to make hydrogen power a commercial success, fuel suppliers must assure hydrogen purity meets SAE standards.
Hydrogen fueled vehicles are popping up around the country. Vehicle manufacturers are offering hydrogen power as a way to achieve low carbon emissions. Unfortunately hydrogen fuel cells are susceptible to damage from impurities commonly found in hydrogen. H2S, ammonia, and carbon monoxide can dramatically shorten the life of fuel cell catalysts. In order to make hydrogen power a commercial success, fuel suppliers must assure hydrogen purity meets SAE standards.
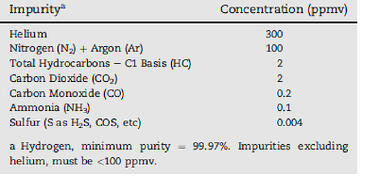
SAE Recommended Levels of Hydrogen Impurities
Detection limits for CO, NH3, and sulfurs are particularly challenging given the tendency of stainless steel and other analytical flow path materials to adsorb or retain active and reactive compounds. Inject a 0.8ppm H2S sample into a 300cc sample cylinder; testing the contents will show complete sulfur loss within hours and even minutes of exposure to the stainless steel surface.
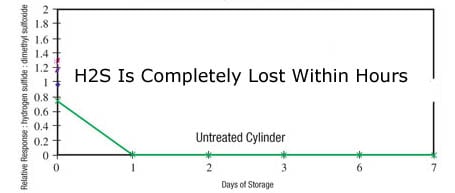
In plant and field test systems, Ammonia, CO and H2S are completely adsorbed into the flowpath surface, making detection and removal of impurities impossible.
Inert coatings like SilcoNert 2000 prevent adsorption of active compounds and impurities, even to part-per-billion detection limits.

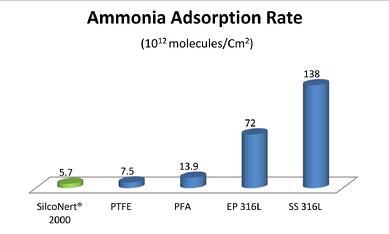
Ammonia adsorption in stainless steel will make detection to the SAE standard difficult if not impossible. SilcoNert 2000 demonstrates the least adsorption among common materials found in ammonia sampling and analytical systems.
Coat the entire sample flowpath. One uncoated fitting can adsorb enough sulfur to significantly alter test results. This can lead to inaccurate results and contamination of fuel cell catalysts.
- Tubing
- Fittings
- Valves
- Regulators
- Fritted filters
- Sample Cylinders

 Hydrogen fueled vehicles are popping up around the country. Vehicle manufacturers are offering hydrogen power as a way to achieve low carbon emissions. Unfortunately hydrogen fuel cells are susceptible to damage from impurities commonly found in hydrogen. H2S, ammonia, and carbon monoxide can dramatically shorten the life of fuel cell catalysts. In order to make hydrogen power a commercial success, fuel suppliers must assure hydrogen purity meets SAE standards.
Hydrogen fueled vehicles are popping up around the country. Vehicle manufacturers are offering hydrogen power as a way to achieve low carbon emissions. Unfortunately hydrogen fuel cells are susceptible to damage from impurities commonly found in hydrogen. H2S, ammonia, and carbon monoxide can dramatically shorten the life of fuel cell catalysts. In order to make hydrogen power a commercial success, fuel suppliers must assure hydrogen purity meets SAE standards.




