
We tested the compatibility of most metals and other materials commonly used in analytical testing and sampling of natural gas. Here's what we found out.
Coating Surfaces for Analytical HPLC and GC
Did you know the base material can have an impact on the performance of CVD coatings? Why is it that we can coat most metals but cannot coat other substrates? To understand why certain metals perform the way they do in our coating process we'll need to jump into alloys and how they react with our coatings.
|
In this blog post you will learn:
How substrate material can affect the performance of silicon coatings.
- How coating temperature can affect metals.
- How metal alloys can improve CVD coating adhesion.
- How some base metal elements cannot be coated but can be coated in an alloy.
- How material compatibility can impact test quality and reliability.
- How to select the best coating and surface for the application.
|

Coating Temperature and Metals
We apply our CVD coatings at temperatures generally around 400°C. This assures a robust bond of our silicon coatings to metal surfaces. Unfortunately some metals can become susceptible to stress corrosion cracking or may be weakened when exposed to higher temperatures. That's the case with some duplex steels, like 2205 and 2507 and aluminum.
The heat of SilcoTek’s CVD coating process can lower duplex stainless steel’s mechanical strength and corrosion resistance. Our coating will still improve the chemical inertness of the surface but may degrade mechanical and corrosion performance depending on the end user’s application requirements.
Coating Aluminum
The physical properties of aluminum (Al) alloys are versatile and ideal for use in a variety of manufacturing or industrial applications. These unique properties, however, can present challenges to surface treatment providers like SilcoTek®. Aluminum is, to some extent, weakened at temperatures in excess of 100° C. Until recently, the aluminum surface also adversely impacted the coating appearance. We developed the SP12 process, a modified SilcoNert® coating application procedure, for improved inertness and corrosion resistance for aluminum and other select materials, as well as to improve the coating appearance.

With this in mind, SilcoTek maintains specifications for processing aluminum parts. SilcoTek does not recommend coating aluminum parts that will be pressurized during use, e.g. sample cylinders. Our processing temperature can weaken the strength of the substrate. The table below illustrates process compatibility with common series of Al alloys:
| Al Alloy |
Compatible |
Incompatible |
| 1000 series |
• |
|
| 2000 series |
• |
|
| 3000 series |
• |
|
| 4000 series |
• |
|
| 5000 series |
|
• |
| 6000 series |
• |
|
| 7000 series |
• |
|

About alloys and why they make a difference in coating results.

An alloy is a mix of a base element metal with another element (or elements). Metal alloys can often have quite different properties than their base metal. In the case of alloys, two wrongs can often make a right. For example aluminum and copper are pretty soft metals, but mix them and you get an aluminum alloy that's stronger.
The same goes for iron mixed with a touch of carbon. Carbon's not even a metal but mix a touch of it with iron and you get steel which can have all sorts of benefits like greater strength. Throw in a bit of chromium into the mix and the resulting stainless steel will vastly improve corrosion resistance.
Alloys have benefits and drawbacks that impact coatability. We'll use nickel as an example.
Confused about what we can coat?
Get our latest quick reference Material Compatibility Guide.
About CVD Coatings: How Material Compatibility Can Impact Performance
Our process is incompatible with pure nickel, because it induces a different growth mode and does not produce the typical nice amorphous (unstructured or non crystalline) coating we see on a stainless steel substrate. You see silicon is happy to bond and diffuse into all sorts of metals, glass or ceramic surfaces but it preferentially does not bond particularly well with nickel.
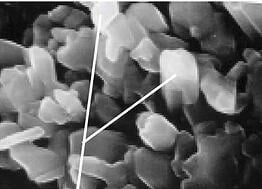
In fact after the first few silicon atoms bond to the nickel surface, the remaining silicon atoms prefer to bond to themselves rather than to the nickel. The result? Under magnification you'll see lots of silicon columns with deep voids, almost like skyscrapers in a big city. That's not a good coating because chemicals you're interested in testing can hide in the valleys, or corrosive chemicals can attack the exposed surfaces. The SEM image below is a general example of what columnar growth looks like under magnification. See? Kind of like big buildings.
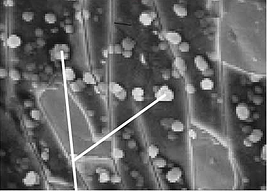
Our amorphous coating when applied to stainless steel, glass, ceramics or many metal alloys will bond uniformly to the surface and build a coating layer-by-layer over the entire surface. It will look something like this SEM image below (note silicon particulates on the surface).
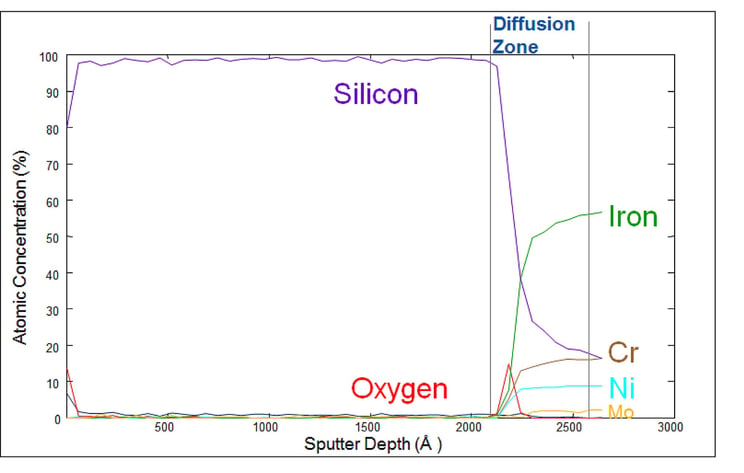
The Auger plot below shows how our silicon barrier coatings bond to the surface. Auger electron spectroscopy (AES) sputter depth profile analysis quantifies the material composition and diffusion zone characteristic of our silicon coatings (in this case our inert SilcoNert® coating). The plot shows a layer of approximately 2000 angstroms (200 nm) of silicon. Note the approximately 500 angstrom diffusion zone between the stainless steel surface and the silicon coating. An Auger depth profile of a coated nickel part would show areas with little to no coating while other areas would have a much greater depth profile.
Back to alloys.
In cases where nickel is alloyed with other “stabilizing” elements, such as Cr (chromium), Si (silicon), P (Phosphorus) etc., even when nickel (Ni) is the major component base metal, our process coats beautifully over these alloys (e.g. Hastelloy®, Inconel, or typical nickel brazing filler alloys such as Ni-Cr-P or Ni-Cr-Si-P). In these cases, the coating is able to bond to the "more beneficial' alloy despite the Ni base metal because the alloy characteristics will allow silicon to more easily or preferentially bond to the surface uniformly. So that's why we can coat superalloys and Ni brazing material.
Incompatibility, The Facts About CVD Coatings
In cases where nickel is alloyed with another difficult to coat element, such as copper, there is no “stabilizing force” or synergy to allow our CVD coatings to bond to the surface. A copper nickel alloy is usually incompatible with our process. Monel is such an example. Nickel and copper by itself are both incompatible with our process, so when you combine these two into Monel, it is incompatible as well.
Conclusion
The data presented shows how the base material can affect the performance of the surface coating. Not only can the base material impact the appearance of the surface, it can have real performance implications. It's important to consider how a base material may interact with any high performance coating or coating process. Our Technical Service Team is here to answer any questions you have about a coating, substrate material or process. We're here to help guide you through the coating process and to help improve the performance of your products and process.
If you want to learn about our coatings and how they benefit your applications, get our webinar:
How To Choose The Right SilcoTek Coating
Have questions about silicon CVD coating material properties? Go to our Material Property Page and get coating specifications, material data and much more. To get the latest in coating technology, follow us on LinkedIn or subscribe to our email and blog.










