
In this blog post we discuss how Dursan improved HPLC performance in metal reactive molecule applications.
Concentrating Metal Reactive Molecules for Pharmaceutical Development
|
In this blog post you will learn:
- How nanoparticles are used in targeted drug delivery applications.
- How Dursan® can alleviate potential HPLC separation problems.
- How an inert barrier coating can prevent surface interaction of reactive materials.
|
Background:
Targeted drug delivery via nanoparticles has been of interest recently as they have the ability to respond to internal and external stimuli. These stimuli include heat, magnetism, and/or chemical interactions. By attaching the molecules of interest to nanoparticles, researchers can deliver drugs to specific locations and release that drug via these stimuli.
The development of these drugs and nanoparticles requires non-trivial analysis as many of the compounds are highly reactive and often are very similar in chemical reactivity and molecular size. Here we will discuss some of these issues and how Dursan® can help alleviate potential separation problems.
Discussion
Utilizing a thiol to link molecules to metallic substrates has been well studied for decades. The use of self-assembled molecular films on solid surfaces via this thiol linkage has attracted widespread interest in a variety of fields for their ability to modify and control topological, chemical, and functional properties at the molecular level. Recently, they have become of interest in medicine as a means to functionalize nanoparticles for drug delivery. Figure 1 is a schematic of the thiol to nanoparticle reaction.
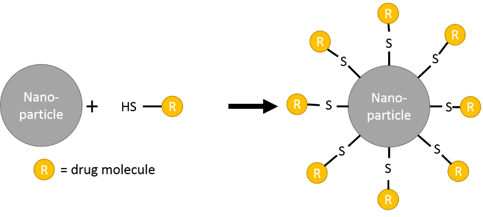
Figure 1: Schematic of the functionalization of a nanoparticle for drug delivery.
Once the molecule of interest is attached to the nanoparticle, it can be injected into the body and directed to a location of interest by modifying the particle size, shape, utilizing magnetism, or by adding surface modifications in addition to the drug molecule additions. Once the particle is concentrated in the area of interest in the body, the drug molecule can be released via a change of temperature, pH, or some other external stimuli.
A recent article in the Journal of Colloid and Interface Science by the Hayes group at Penn State’s Department of Biomedical Engineering investigated utilizing a retro-Diels-Alder reaction for the release of short non-coding nucleic acid molecules from silver nanoparticles.
As can be seen in Figure 2, once the molecule is synthesized and attached to the nanoparticle, heat can be used to activate the retro-Diels-Alder reaction to release the drug molecule. By changing the atom in the cycloadduct created by the Diels Alder reaction, the researchers found that the release rate and temperature at which the release occurs can be controlled. This can allow scientists to design nanoparticles that are capable of delivering multiple drugs that can be released at different times for gene therapy just by utilizing a different temperature for the release.
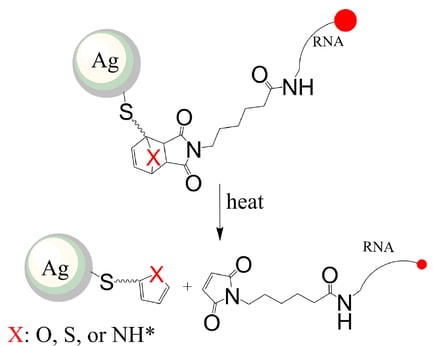
Figure 2: Release of the therapeutic RNA molecule occurs via the retro-Diels-Alder reaction. It was found that changing X results in different release rates at different temperatures.
Why use Dursan CVD Coating Technology in HPLC Analysis?
The synthesis of these thiol linkers is not difficult, but the purification and concentration of the molecules can be troublesome. HPLC is of interest due to its ability to separate compounds quickly and with high accuracy. In the case of these linker molecules, they are quite sensitive, especially to metals, and a typical stainless steel or titanium HPLC system will not be able to efficiently separate the molecules of interest for purification.
Dursan is a non-reactive, inert, and corrosion resistant barrier coating that can be applied to high tolerance components and to interior bore of small diameter tubing. Dursan can even be applied to metal fritted filters, preventing chemical reactivity and adsorption while maintaining filtration efficiency.
Learn more about how SilcoTek® coatings improve HPLC performance. Get our e-book.

Figure 3 shows the three linker molecules that were used in this study. Highlighted are areas of the molecules that pose issues for metal components in an HPLC system. These areas will have strong interactions with the metal and cause significant tailing and broadening of peaks as well as the complete loss of molecules via adsorption. In the world of pharmaceutical production, this is not acceptable. By coating the flow path with Dursan, the adsorption and metal interaction with stainless steel systems can be avoided.
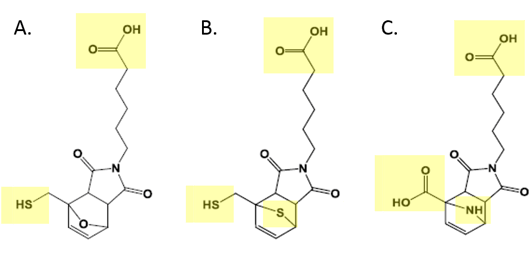
Figure 3: A. Furan B. Thiophene and C. Pyrrole linkers used to attach the short non-coding nucleic acid molecules to silver nanoparticles. The carboxylic acid attached to the cycloadduct on the pyrrole was further modified by crosslinking cysteamine using EDC coupling chemistry to create the thiol for nanoparticle binding. The carboxylic acids at the end of the alkyl chains are modified utilizing the EDC coupling to N-hydroxysuccinimide to create an amine terminated molecule for RNA attachment.
Figure 4 shows a sample chromatogram of the separation done to isolate the furan linker. The last peak is the furan linker and is the molecule the group was attempting to isolate and concentrate for further characterization. The researchers commented that the peaks shapes are not optimized for peak efficiency. They were optimized for clean separations. The injection volumes and concentrations were also quite high as the group was attempting to minimize the number of separations needed to concentrate their product. Without the Dursan coating, these molecules would react with the walls of the HPLC column rather than the clean separation seen here.
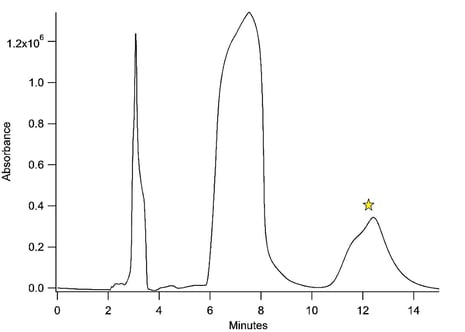
Figure 4: Chromatogram of the furan linker separation. The peak with the star over it is the peak of interest for this separation. All additional peaks are byproducts and side reactions from the synthesis of the linker molecule.
Conclusion:
Dursan provides an inert barrier between metal substrates and highly reactive molecules such as the nanoparticle linkers shown above. This inert barrier is important, especially in a pharmaceutical setting where the molecule must be isolated and concentrated quickly and with high accuracy. This experiment uses silver, but iron oxide could be used as well. The nice part about iron oxide is it’s magnetic, so targeted drug delivery may be accomplished by applying a magnetic field.
An author that worked with the Hayes group commented, “The group that was using the coated column to isolate and purify their sensitive novel compounds have completed their study. The LC work was critical as it allowed further characterization of the compounds they synthesized.”
Have a question about our coatings or would like to use our coatings in your testing or product evaluation? Contact our Technical Service Team and we'll discuss your application and recommend the best coating for your application.

Reference:
Abu-Laban, M.; Kumal, R. R.; Casey, J.; Becca, J.; Lamaster, D.; Pacheco, C. N.; Sykes, D. G.; Jensen, L.; Haber, L. H.; Hayes, D. J. Journal of Colloid and Interface Science 2018, 526, 312–321.







