
A recent paper published in ACS Energy Fuels discusses the link between the quality of mercury analysis and exposure to trace sulfur compounds in natural gas analysis.
The Impact of Trace Sulfur on Mercury Analysis
Refinery and natural gas samples often contain trace amounts of sulfur containing compounds like hydrogen sulfide (H2S) and mercury containing compounds. Unfortunately the presence of sulfur, hydrogen sulfide and other sulfur compounds can interfere with mercury analysis and result in degraded test performance.
Have a Question? Contact Our Technical Service Team
In this blog post we'll discuss how sulfurs can impact the quality of mercury analysis.
|
In this blog post you will learn:
- How various surfaces perform under trace mercury analysis conditions.
- How trace sulfur exposure can impact mercury adsorption and stability.
- How used sample cylinders can alter trace mercury test results.
|
A recent paper published in Fossils & Fuels* (a journal from the American Chemical Society) shed light on the impact of trace sulfurs have on mercury analysis in natural gas, the results indicate potentially degraded mercury test performance due to adsorption in used sample cylinders. The paper abstract states the following:
“We find that gaseous Hg concentration sharply declines in almost all tested cylinders (uncoated, PTFE-coated and silicon-coated) to reach undetectable levels within a day or two due to adsorption, with the notable exception of a brand new silicon-coated cylinder. Heating cylinders up to 190 °C allowed the recovery of most of the adsorbed Hg and revealed the occurrence of two distinct Hg species with distinct release temperatures. Our results suggest Hg0 is first physically adsorbed and further oxidized, presumably in relation to sulfur compounds covering the internal walls of the cylinders. The newly purchased silicon-coated cylinder kept a constant gaseous Hg concentration over 6 months because it never interacted with any real Natural Gas sample containing substantial sulfur concentrations relative to Hg.”
The initial adsorption rate of mercury fits the pattern of inertness, with SilcoNert® coated cylinders offering superior performance compared to uncoated or PTFE lined cylinders. The graph below (found in the paper), compares the initial mercury adsorption rate of various surfaces.
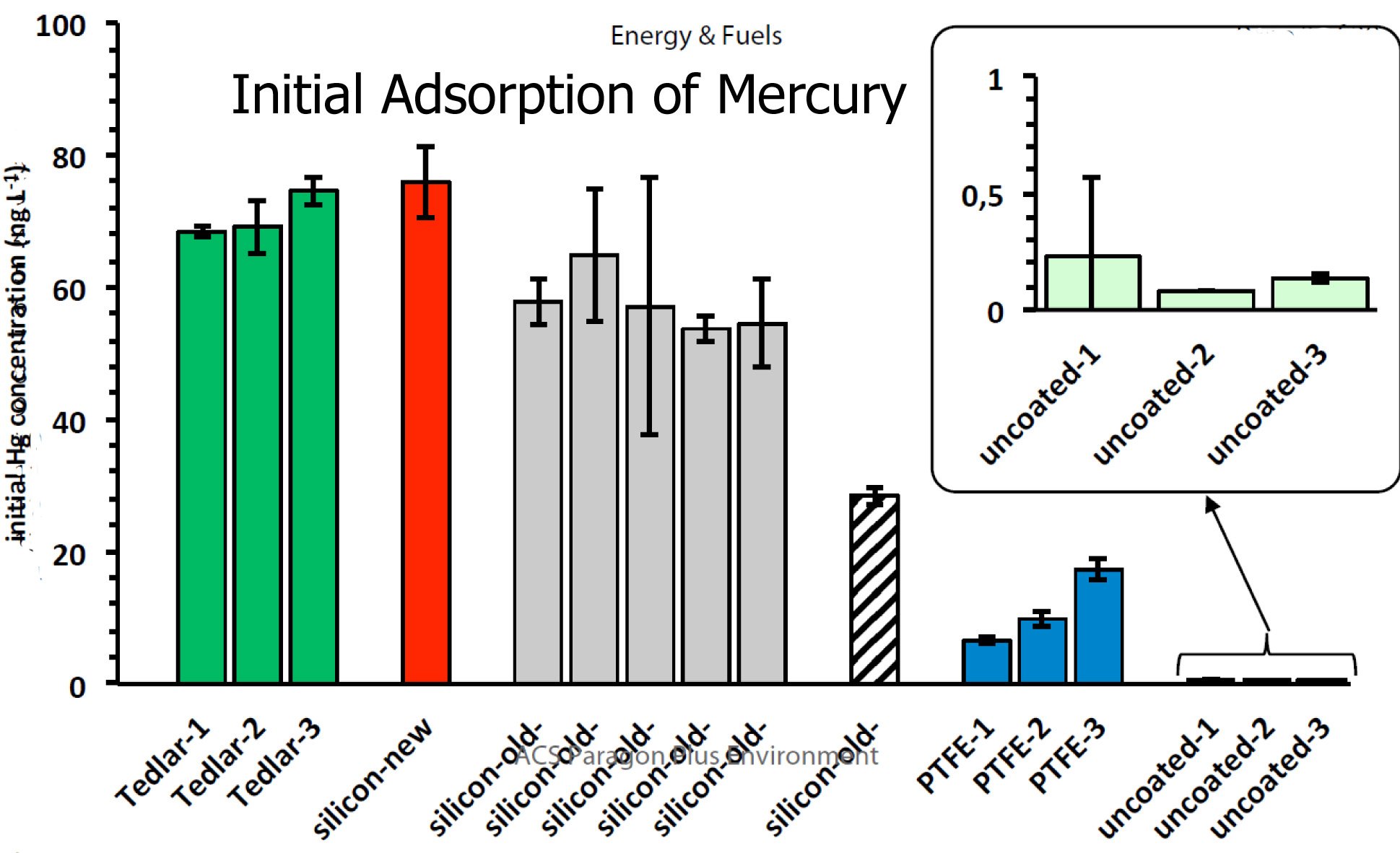
Comparison of new vs. old surfaces (previously exposed to natural gas containing sulfurs) shows a reduction in mercury test performance. The new SilcoNert coated surface outperformed other surfaces. (Significantly in most instances).

Further long term testing showed a distinct drop reduction in mercury that was able to be restored by heating the surface. The results indicate possible mercury adsorption by surfaces previously exposed to sulfur containing natural gas. The paper summary states:
"Heating cylinders up to 190 °C allowed the recovery of most of the adsorbed Hg and revealed the occurrence of two distinct Hg species with distinct release temperatures. Our results suggest Hg0 is first physically adsorbed and further oxidized, presumably in relation to sulfur compounds covering the internal walls of the cylinders."
Want to learn more about the study? Read the complete mercury paper.

This is likely very important information for analysts testing mercury in oil and natural gas applications. If the analyst uses a sample cylinder that has been exposed to sulfur compounds, their mercury analysis results may be degraded. A new SilcoNert® coated surface performs exceptionally well in mercury test applications. Previous testing by SilcoTek® has shown the SilcoNert coated surface will maintain mercury stability for up to 50 days. Read more about SilcoNert and mercury testing.
/mercury%20analysis.JPG.png?width=661&name=mercury%20analysis.JPG.png)
Cleaning the exposed surface may minimize mercury adsorption, as always it's prudent to test sample cylinders or surfaces with the appropriate chemical standard (in accordance with company and industry standards) to verify performance in the application before using the cylinder or flow path surface in the field.
Have a question about mercury analysis? Contact our Technical Service Team or follow us on LinkedIn.

*Experimental tests of natural gas samplers prior to mercury concentration analysis
Maxime Enrico, Aurore Mere, Honggang Zhou, Herve Carrier, Emmanuel Tessier, and Brice Bouyssiere
Energy Fuels, Just Accepted Manuscript • DOI: 10.1021/acs.energyfuels.9b03540 • Publication Date (Web): 26 Dec 2019




/mercury%20analysis.JPG.png?width=661&name=mercury%20analysis.JPG.png)

