
What's the fastest surface? We challenged some of the best known analytical flow path materials to a race. Here's what happened.
I've been running for years but I've gotta say that I'm not the fastest runner. When you get passed by people pushing babies or grandpas in walkers you know you're not the fastest in a foot race. That's why when someone challenges me to a race I'm not likely to place a wager on me beating anyone capable of mustering more than a snail pace. But when it comes to coatings and fast material surfaces, I'll bet the farm on SilcoTek® every time. (OK, I don't have a farm but you get my drift...) So it's put up or shut up time on declaring who's got the fastest analytical flow path surface. This is ridiculous you say? Well maybe but it's freezing outside, so what else do you have going on? Complete disclosure, we're not talking about actually racing the materials, that would be a looong race. No, we challenged some commonly used analytical instrument materials (in 1/4 & 1/8th inch tubes) to see how fast reactive and sticky compounds raced through the tubes. Who will contend for the title of fastest analytical material?

The Contenders
–PTFE
–Stainless steel 316L
–SilcoNert® 2000, our CVD silicon coating, applied to 316L stainless steel.
The Race Track
We chose a neutral site. Sensors Inc. set up the conditions and test chemical. They chose ammonia as the standard and 1/4 and 1/8th inch tubes as the track.

The Test
- Sensors Inc., Testing uptake and release of ammonia through a sampling system
- Comparison of 3 different ¼” and 1/8” sample lines. 3 meter length
- 11.5 ppm ammonia (NH3)
- Flow rate, 10 L/hr
- Room temperature
The Race
- Uptake Test : Time to wet the sample path length. We'll measure how long it takes for the sample to travel through the tube. If the tube surface adsorbs or impedes flow, it will take longer for the sample to hit the detector finish line. So the less time through the tube the better.
–Desired results: The winner will have no lag or delay.
The benefit?
- Create shortest possible cycle time for instrument reading and optimum process control. (Sample quickly moves to detector)
- No sample loss in inert transfer assures all sample gets to detector
- Fast Release: Time to return to baseline fast with no noise.
- Eliminate potential interference by cross contamination or carryover. If all the sample gets through the tube, none will be left to contaminate the next sample.
- “Memory” of sample created by hold up on active sites will be eliminated.
- Create shortest possible cycle time
- Faster return to baseline, faster next test can start
|
What Material is Fastest for Analytical Response? The Winner is SilcoNert!
|
Sampling Line Material
|
Average Rise Time
(average of 5 replicates)
|
|
PTFE
|
16.2 seconds
|
|
SS316L
|
17.0 seconds
|
|
SilcoNert® 2000 coated 316L
|
8.4 seconds
|
SilcoNert® 2000 coated tubing shows improved release over other alternatives
How about we try that again, but this time we measure the time it takes to release or purge the system of ammonia. Ready set go!
|
Sampling Line Material
|
Average Fall Time
(average of 5 replicates)
|
|
None (intrinsic analyzer response)
|
6.0 seconds
|
|
PTFE
|
18.2 seconds
|
|
SS316L
|
33.0 seconds
|
|
SilcoNert® 2000 coated SS316L
|
9.4 seconds
|
Wow! 2 for 2, SilcoNert® 2000 coated tubing shows improved release over other alternatives.
Get all the details on the ammonia inertness comparison.
OK let's go double or nothing. This time testing for sulfur adsorption.
Lets run a trace amount of methyl mercaptan through a 100ft length of 1/8 in diameter tube and see who comes out the other end first.
Stainless steel goes first. After over 90 minutes, our finish line detector finally responds, signaling that the stainless tube adsorbed / retained the mercaptan for a significant period. This kind of performance could impact analyzer response and can lead to process upsets or regulatory compliance issues. A real loser in my opinion.
Next up SilcoNert® coated electropolished stainless steel. That super inert surface is intimidating but let's see what it's got on the field. Wow let's check the replay! The SilcoNert surface blew away the competition by hitting the finish line detector in about 3 minutes! Here's the results.
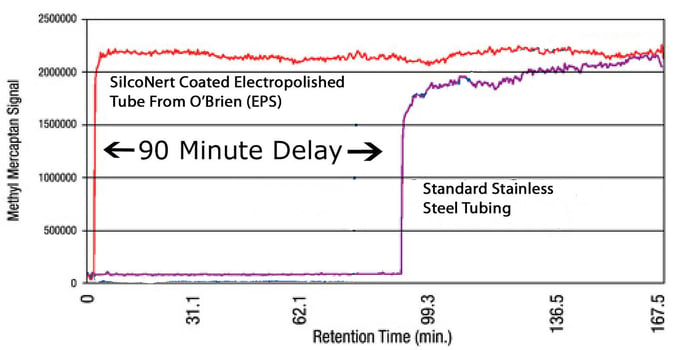
Winner SicoNert! By a huge margin SilcoNert offers faster response compared to stainless steel or PTFE. In fact SilcoNert beat stainless steel by over 90 minutes. Now that's fast.
Read the complete study on the sulfur analysis comparison.

Why is this race important and why speed matters.
Fast, reliable sample results with minimal adsorption or contamination means better process control, improved monitoring of critical processes and more accurate data for regulatory compliance. Knowing exactly what you're sampling and when it was taken can improve product yield and avoid shipping sub-standard product to downstream customers. So why is speed important? It all comes down to saving money by avoiding sample problems.
Looks like I'll be challenging stainless steel and PTFE to a race. It seems to be the only thing I can beat.
Learn how you can beat ammonia and sulfur hands down! Go to our solutions page.







