 *
*
If you're familiar with the SilcoTek® website and blogs you know that we offer inert coatings. But what does that mean? A web search will tell you that an inert substance is not chemically reactive, has no ability to react, or does not produce a chemical reaction. That sounds suspiciously absolute to me.
 I guess I inherited some of my mother's skepticism. Like when I told my mom I absolutely did not try to burn down the garage. At which point my mother looked down her glasses at me and said "really, you're absolutely sure you had nothing to do with trying to burn down the garage. You had nothing to do with the candle, the batteries, absolutely nothing to do with it." At which point the nuance of the situation made me wonder if I absolutely had anything to do with the "incident". So I fessed up that I may have had something to do with it. After all what in life is ever absolute? As with that "incident" which I will never discuss again (due to statute of limitation issues...) the absolute statement of being non reactive or no ability to react seems a bit fishy.
I guess I inherited some of my mother's skepticism. Like when I told my mom I absolutely did not try to burn down the garage. At which point my mother looked down her glasses at me and said "really, you're absolutely sure you had nothing to do with trying to burn down the garage. You had nothing to do with the candle, the batteries, absolutely nothing to do with it." At which point the nuance of the situation made me wonder if I absolutely had anything to do with the "incident". So I fessed up that I may have had something to do with it. After all what in life is ever absolute? As with that "incident" which I will never discuss again (due to statute of limitation issues...) the absolute statement of being non reactive or no ability to react seems a bit fishy.
So what is an "inert surface" and how do I know if I have one?
Digging further into the definition you'll find the term gets less clear. You'll find that given enough energy and under the right conditions almost anything will react. It's all about the conditions and specific chemicals under which the surface should be non reactive. So be weary of claims of absolute inertness.
One other key factor to consider is surface adsorption. A flowpath may not react chemically with a target compound, but will it allow other substances to stick or adhere to the surface? Adsorptive surfaces will retain a substance on the surface, creating a film of adsorbate that will eventually desorb or be released back into the sample stream. Adsorption is caused by physisorption (van der Waals forces), chemisorption (covalent bonding), or electrostatic attraction. Adsorption can be just as important a factor as surface inertness.
Here are 3 tips to determine if a surface is reacting with your sample.
1. Test for the relevant target compounds.
If a manufacturer claims a surface is non reactive but does not produce data relevant to your application, test the surface under controlled conditions to be sure the surface is truly not reacting with your compounds. Test both adsorption (loss of compounds) and desorption (gain of compounds) in a stream.

Here are 2 examples of how to test for surface reactivity and desoption. Example 1 is a comparison of a coated and uncoated 1/8in tube. Take a 100ft length and coil it. Flow a sample of your analyte (in this case sulfur in gas form) through the tube. Analyze the output to determine if the output matches the known input concentration. Data courtesy of Shell Corporation & O'Brien Corporation.
To determine desorption or retention of your target compound, flow nitrogen or other "non reacting" gas through the tube and analyze the output for your target analyte. (example 2)
|
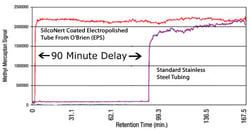
Example 1
Adsorption can delay or prevent accurate testing. Reactive surfaces like stainless steel can significantly impact results.
|
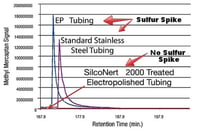
Example 2
Desorption or delayed release of compound from a reactive surface can lead to false or misleading results.
|
2. Contact angle measurement.
Surface energy can play an important role in retention of compounds. Easily wet surfaces with low contact angle and high surface energy (left image) may react more readily with target compounds. Low energy surfaces that don't wet and produce a high contact angle (right image) may be less reactive to your analyte.
|
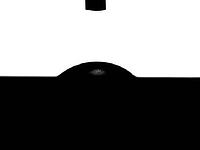
High energy, low contact angle, wetting surface may be reactive.
|

Low energy, high contact angle, non wetting surface may be less reactive
|
3. Heat is your friend.
If you really want to know if a surface flowpath non reactive, test it under elevated temperature. A heated surface will increase the rate of chemical reaction and will bring out the worst in a surface. Always test surfaces under the same temperaure conditions as in the field.
Want more details on how to prevent surface interaction? Read our presentation and learn about the benefits of a non reactive flowpath.

*Image credit: http://chemistry.about.com/
**Image credit: http://www.wspynews.com/
 *
*






