This week, we wanted to share a real world example that comes from within. A SilcoTek® employee had a baby girl who was errantly diagnosed with hyperthyroidism at birth due to suspected analyte carryover. Our research and development team took a look at how this could have happened, and a possible way to prevent it in the future.
Newborn children have their blood tested for a variety of conditions that are treatable, but not clinically evident in the newborn period. This blog post will discuss the tests that are done and discuss the potential for misdiagnosis via a story from a SilcoTek employee. The utilization of SilcoTek coatings can minimize the potential for such misdiagnoses that are a result of analyte carryover from sample to sample.
For most newborns in the United States, a nurse will immediately make a small prick to the bottom of the child’s foot and collect blood samples to screen for a variety of diseases. These diseases are ones that typically do not present any symptoms in newborns but are treatable if detected early. Examples of such diseases include:
- Phenylketonuria (PKU): an inherited disease in which the body cannot metabolize a protein called phenylalanine, which naturally occurs in most protein-rich foods like milk, eggs, and meat.
- Congenital hypothyroidism: a condition where the baby is born with too little thyroid hormones which can lead to metabolic issues.
- Galactosemia: an inherited disease where the body cannot metabolize galactose, a sugar found in milk.
- Sickle cell disease: an irregularity in hemoglobin causes red blood cells to be misshapen and not effectively carry oxygen to the body. Clinically this disease is not spotted until 6-12 months after birth.
A variety of tests are used to detect issues above. For instance, PKU tests used to be done via bacterial inhibition assays where the blood is exposed to a bacteria that will only grow in the presence of phenylalanine. You can see a video describing this test here. The problem with a test like that is one test can detect one condition. With advances in modern technology, lab technicians can screen for many diseases with just a small amount of blood sample. Clinical testing laboratories often use equipment called immunoassay analyzers to test for a variety of conditions from a single blood sample. In vitro diagnostic (IVD) equipment manufacturers, such as Abbott Laboratories, Beckman Coulter, and Roche Diagnostics, make immunoassay analyzers than can handle thousands of samples per day. These companies work hard to ensure the analysis is robust and reliable. A major issue for all IVD equipment is the potential of false readings such as false negatives due to the analyte of interest getting trapped or stuck along the flow path and/or false positives which are typically due to carryover. Here is a nice article that discusses potential sources of carryover. A more recent editorial about sample carryover can be read here.
The carryover effect can be due to a variety of issues such as poor washing of reusable components or as SilcoTek has found in its studies, metal surfaces can cause issues in IVD instrumentation. This post will discuss a specific example of carryover experienced by a SilcoTek employee shortly after the birth of his daughter.
Data and Discussion
Hyperthyroidism is a condition where the thyroid, a butterfly shaped gland around the throat, over produces certain hormones. This condition impacts roughly 1.2% of the American population. About half of these cases have obvious symptoms, while the other half do not. It is also far more common in women than in men. Symptoms often do not show until later in life, so a test of a newborn is done for elevated levels of triiodothyronine (T3) and thyroxine (T4). These two compounds are hormones secreted from the thyroid and could lead to developmental issues if left untreated in newborns. Catching high levels of these compounds early leads to treatments that can ease these issues later in life. The chemical structure of T3 and T4 can be seen in Figure 1. Highlighted on both compounds are carboxylic acid groups. These carboxylic acid groups are well known to chelate to metals in solution and metal surfaces. T3 has been shown in studies to bind to copper ions in solution. This binding helped to protect low-density lipoproteins from oxidation, as copper ions are Lewis acids in solution and will readily oxidize materials like proteins or lipids. The study showed that T3 and its acetic derivative not only protected the low-density lipoproteins, but they also protected them better than butylated hydroxytoluene (BHT), a free radical scavenger, and ethylenediaminetetraacetic acid (EDTA), a well known metal chelating agent.
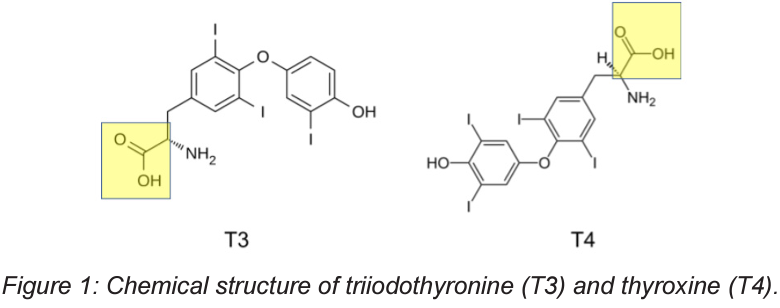
Figure 1: Chemical structure of triiodothyronine (T3) and thyroxine (T4).
In the spring of 2018, a SilcoTek employee had a baby girl. Like any other baby, a small foot prick was immediately performed and sent for analysis. It was reported that the child had elevated T3 hormone in her blood which indicated the possibility of hyperthyroidism. The child did not show any symptoms of hyperthyroidism such as irritability, low weight, high body temperature, high heart rate, or difficulty sleeping. With no symptoms and no hereditary history of hyperthyroidism, a second test with a larger blood sample was performed. The results showed no signs of hyperthyroidism as all thyroid hormones were within normal ranges.
At a follow-up appointment with the child’s doctor, the SilcoTek employee asked, “So was the first test just off? What happened?” It turned out that there were four children all born on the same day at the hospital. All four of them tested positive for hyperthyroidism with high T3 counts. After follow-up tests it was found that the child tested first was confirmed to have high T3 counts, while the following three children had hormone levels in the normal range. It is unknown as to whether the carryover was a result of insufficient probe washing (which are made of metal) or due to metal in the flow path other than the probes, but the original positive case contained enough T3 in the sample to cause carryover to the subsequent three tests, resulting in “above normal” range for each test.
Figure 2: Misdiagnosed child of a SilcoTek employee immediately after the blood draw for a follow-up test.
In summation, IVD instruments and their metallic components have the potential for carryover effects that can have real world impacts to patients. Here we discussed a newborn screening where a carryover event caused 3 families to receive a false positive of high T3 counts which often indicates hyperthyroidism. Follow-up tests showed that the original measurement for 3 out of 4 infants was an error, whether that be due to wash insufficiencies or metal sites in the flow path causing the carryover. Coating metal components in such instrumentation with Dursan® would provide higher test reliability, as it would not allow the metal active analytes to be retained in the analytical flow path.
SilcoTek's Dursan coating can solve many problems, including:
-
Poor performance caused by carryover of proteins
-
High costs due to corrosion, especially from bleach
-
Contamination from leaching of metal ions out of equipment
-
Poor analytical sensitivity for challenging samples
-
Recurring replacement because of material wear
We commonly coat many diagnostic tools, such as:
-
Needles & Syringes
-
Nozzles
-
Pump heads
-
Metal fritted filters
-
Stainless steel tubing
-
Ampoules
-
HPLC columns
-
Valves and Fittings
-
Clinical chemistry and immunoassay analyzers
-
Mandrels, plungers, extrusion tips, dies
-
Chemical vessels & containers
-
Sensor probes
-
Blood Centrifuges
-
Cannulas

Think your application could benefit from SilcoTek's barrier coatings? Contact us for a free consultation with our experts!






