
We tested the corrosion resistance of Dursan® in TFA, a common ion pairing agent in the analysis of peptides and small proteins in HPLC testing. Is Dursan resistant to TFA in HPLC testing applications? Here's what we found out.
Trifluoroacetic acid (TFA) is a structural analogue of acetic acid where all three of the acetyl hydrogens are replaced by fluorine atoms as seen in Figure 1. 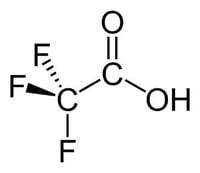 TFA is a much stronger acid than acetic acid due to the electron withdrawing nature of fluorine to weaken the oxygen-hydrogen bond. It is commonly used in low concentrations as an ion pairing agent in the analysis of peptides and small proteins in liquid chromatography. Dursan can be used in liquid chromatography environments to provide an inert coating to the surface of stainless-steel components such as frits, columns, pumps, and tubing. This blog post will investigate the compatibility between Dursan and TFA and get ideas for Solving HPLC Test Problems.
TFA is a much stronger acid than acetic acid due to the electron withdrawing nature of fluorine to weaken the oxygen-hydrogen bond. It is commonly used in low concentrations as an ion pairing agent in the analysis of peptides and small proteins in liquid chromatography. Dursan can be used in liquid chromatography environments to provide an inert coating to the surface of stainless-steel components such as frits, columns, pumps, and tubing. This blog post will investigate the compatibility between Dursan and TFA and get ideas for Solving HPLC Test Problems.
|
In this blog post you will learn:
- The compatibility of Dursan and TFA
- Corrosion rate of Dursan when exposed to TFA solution
- Stiction of TFA to Dursan vs. stainless steel.
- Application of Dursan in HPLC
|
The Test
Corrosion coupons were coated with our standard Dursan® coating. They were then subjected to an immersion test as specified by ASTM G31 methodology. TFA is typically used in protein analysis at concentrations of ~0.1%. The primary area of concern for the Dursan coating is corrosion which is typically accelerated at higher concentrations. For this study we chose to expose coupons to 1% and 2% TFA solutions for 30 days at room temperature. Film thickness, coupon mass, and water contact angle were monitored before and after the 30-day exposure.
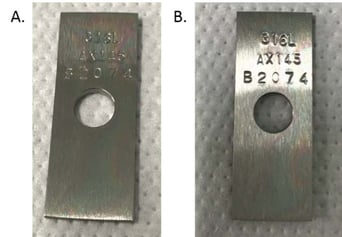
Thickness was measured on 5 points of each coupon prior to and after the 30-day exposure. Results are summarized in Table 1. There was no appreciable loss of the Dursan coating due to the TFA exposure. Figure 2 also shows a before and after picture of one of the coupons exposed to the 2% TFA solution. There is no change in appearance. The slight change in thickness is due to not measuring the exact same spot on the coupon before and after exposure. With the slight variation in film thickness that happens naturally as a result of the deposition conditions, the values appear to change, but they are all within the error of the measurement. Want to learn more about how our coatings can improve HPLC performance? Get our HPLC Application Guide.

Table 1: Comparative thickness of a Dursan coated coupon before and after 30 day exposure to TFA.
|
TFA concentration
|
Average thickness before exposure
|
Average thickness after exposure
|
|
1%
|
1135 ± 28 nm
|
1129 ± 29 nm
|
|
2%
|
1128 ± 25 nm
|
1139 ± 30 nm
|
Figure 2: Dursan coated corrosion coupon before (A) and after (B) 2% TFA exposure for 30 days. There is no visual difference between the appearance of the coupon.
Contact Angle Measurement
Finally, contact angle was monitored to investigate the surface chemistry before and after the exposure to TFA. The Dursan coupon had a water contact angle of ~90° prior to the exposure. This is typical for Dursan. After the 30-day exposure to both 1% and 2% TFA, the water contact angle decreased to ~55°. This is similar to what occurs when the coating is exposed to a bleach solution for 30 minutes.
Regardless of this change in contact angle, the Dursan surface still shows remarkable anti-fouling properties for proteins. The bare stainless steel coupon started with a contact angle of ~35°, and after the exposure to TFA, a DI water rinse, 30 minute sonication in DI water and dried at 230°C, the coupons had a contact angle of ~110°C. This suggests that the TFA sticks to the bare stainless steel readily, and is difficult to remove, which is a well-documented problem with TFA in HPLC applications. The Dursan coupons (which saw the same post exposure conditions) show that TFA does not adhere well to the surface, which is an added benefit when using Dursan coated HPLC components for protein and peptide analysis. Want more details on how Dursan can improve HPLC test results?

Dursan Use in HPLC
Dursan® can be applied to most stainless steel HPLC flow path surfaces, improving corrosion resistance in inertness of HPLC systems. Apply Dursan to:

- Stainless steel tubing
- Valves and fittings
- Columns
- Needles
- Glassware
- Ampules and vials
In conclusion, TFA poses no risk to Dursan coated parts. There is no change in thickness of the coating, and no corrosion occurs of the underlying substrate (in this case 316L stainless steel) during a 30-day exposure to 1-2% TFA solutions at room temperature. The contact angle of the coating does change to become more hydrophilic; however, previous studies have shown that this has little to no effect on the anti-fouling properties for protein analysis. An added benefit of the Dursan coating is that it appears to not allow the TFA to stick to the surface of the coupon, where a bare coupon has evidence of TFA stuck to the surface even after rinsing and sonication of the coupon.
Have a question about a coating application?
Ask the experts.

Go to our FAQ page, or contact our Technical Service Team. We're here to help you select the best coating for your application and keep your coating surface running at peak performance.
Our team can:
- Recommend the best surface preparation, coating maintenance, and test methods for your application.
- Offer the latest coating test data that best fits your application.
- Team up with your R&D staff to perform evaluation testing and product optimization.
- Offer test samples at no charge.

 TFA is a much stronger acid than acetic acid due to the electron withdrawing nature of fluorine to weaken the oxygen-hydrogen bond. It is commonly used in low concentrations as an ion pairing agent in the analysis of peptides and small proteins in liquid chromatography.
TFA is a much stronger acid than acetic acid due to the electron withdrawing nature of fluorine to weaken the oxygen-hydrogen bond. It is commonly used in low concentrations as an ion pairing agent in the analysis of peptides and small proteins in liquid chromatography. 




