
In part 4 of our metal ion leaching series we test metal ion leaching in high purity deionized water. Will coatings prevent DI water metal ion leaching in HPLC systems?
Preventing Metal Ion Leaching in DI Water
Corrosion encompasses a large variety of material degradation. It can be large enough to see visually such as the numerous examples in harsh acids or salt fog chamber experiments that SilcoTek® has performed, or corrosion can be at trace levels that are not detected visually or by weight loss but none-the-less can affect analytical test results or processes through metal ion leaching.
We previously investigated Titanium, 316 Stainless Steel, and C-22 Hastelloy in pure methanol and monitored their corrosion (exhibited by metal ion leaching) via ICP-MS. Here we look at ultrapure DI water to see if the corrosion patterns follow the methanol studies.
|
In this blog post you will learn:
- How effective are SilcoTek coatings in identifying and preventing metal ion leaching and contamination in high purity DI water.
- How to test various coatings for trace corrosion resistance.
- How SilcoTek coatings performed when applied to titanium, Hastelloy®*, and stainless steel surfaces.
|
Background:
Previous blog posts have investigated metal ion leaching on common alloys for HPLC, analytical and other industrial process applications. So far, we have investigated water and methanol’s impact on stainless steel, titanium, and C-22 Hastelloy. Part one through three of this study can be found below:
In previous ion leaching studies, sintered discs made of the various alloys were exposed to pure methanol for one month. The resulting solution was then examined via inductively coupled plasma mass spectrometry (ICP-MS) for metal ion contamination. The results showed that all three coatings provided by SilcoTek® were able to stop metal ion contamination for 316 SS and C-22 alloys. Only Silcolloy® was able to provide protection for titanium ion leaching. The reasons for that were discussed in part 2 of this series.
Here we use the same experimental set-up to investigate DI water’s impact on these three alloys. Among numerous other uses, DI water is commonly used as a solvent for HPLC analysis, in food and drug production, and as a precursor in semiconductor applications. Here we show that titanium alloys do not suffer metal ion leaching in DI water, while both stainless steel and Hastelloy show large amounts of metal ion leaching, specifically an abnormally large amount of nickel leached into DI water when compared to the methanol leaching studies.

Data and Discussion:
 Porous 316 Stainless Steel and C-22 Hastelloy porous discs were purchased from Mott Corporation. The titanium porous discs were purchased from VICI precision sampling. All porous metal discs used in this study have a nominal pore size of 10 µm. One disc of each alloy type was coated with Dursan®, Silcolloy®, and a new test coating we're calling RD5. Each coated disc and a bare disc were placed in separate polypropylene containers with 50 mL of UHPLC grade DI water purchased from MilliporeSigma. Additionally, one polypropylene container was filled with 50 mL of water with no disc in it to act as a blank or control for the study. After one month of soak time, the porous disks were removed from the water and the samples were delivered to the Energy and Environmental Sustainability Laboratories at Penn State University. Samples were then prepared by addition of nitric acid to achieve the correct pH and were then analyzed using a Thermo Fisher iCap RQ ICP-MS.
Porous 316 Stainless Steel and C-22 Hastelloy porous discs were purchased from Mott Corporation. The titanium porous discs were purchased from VICI precision sampling. All porous metal discs used in this study have a nominal pore size of 10 µm. One disc of each alloy type was coated with Dursan®, Silcolloy®, and a new test coating we're calling RD5. Each coated disc and a bare disc were placed in separate polypropylene containers with 50 mL of UHPLC grade DI water purchased from MilliporeSigma. Additionally, one polypropylene container was filled with 50 mL of water with no disc in it to act as a blank or control for the study. After one month of soak time, the porous disks were removed from the water and the samples were delivered to the Energy and Environmental Sustainability Laboratories at Penn State University. Samples were then prepared by addition of nitric acid to achieve the correct pH and were then analyzed using a Thermo Fisher iCap RQ ICP-MS.
The results for the titanium discs can be seen in Figure 1. All values were within the detection limit of the instrument for titanium (0.2 ppb) showing that in ultrapure DI water, no titanium ions leach into the solution.
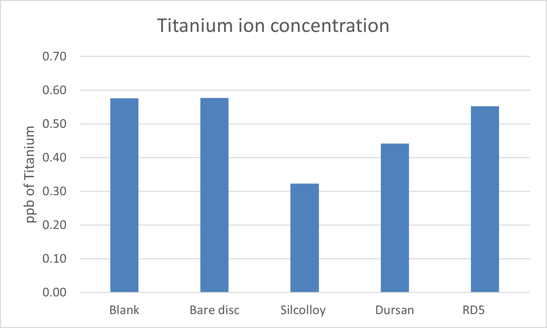
Figure 1: Titanium ion leaching in pure DI water. Blank refers to no sintered disc placed in the liquid to act as a control. All samples showed no titanium ion leaching into solution.
This is strikingly different than the methanol study where 14 ppb of titanium were leached into the methanol. Dursan and RD5 seemed to exacerbate these results (21ppb and 57 ppb respectively).
Previous studies have shown that if titanium is exposed to water, it will passivate the surface and not allow the pitting of the titanium surface and the release of ions into the solution.1 This also explains observations of De Pra and his group at Thermo Fisher Scientific where they investigated titanium contamination of their HPLC system.2 They found that the silica bed in the column can be poisoned with titanium if using pure methanol and a titanium sintered filter in the solvent bottle. Switching the solvent from pure methanol to just 5% water was sufficient to prevent the metal ion leaching into their system.
Learn more about how our coatings can improve the performance of HPLC and other analytical systems. Click the image below to read our Applications Guide.

The 316 stainless steel disc results can be seen in Figure 2. The coatings on the sintered metal discs block the metal ions from leaching into solution. While there was substantial metal ion leaching into the solution, it was far less than experienced in the previous studies using methanol. Specifically, the iron and chromium levels were roughly an order of magnitude lower, as shown in Table 1.
The nickel concentration is relatively unaffected by the solvent choice. That's because the surface of 316 stainless steel has an oxide layer, largely consisting of chromium oxide, but also containing iron and nickel oxides. This layer provides a protective surface and is often self-healing when there is an oxygen source, such as air or water.
In this case, it can be concluded that the comparatively low levels of chromium leached into the water indicates less damage to the protective oxide layer when compared to pure methanol. With less damage to the oxide layer, there is less access to the iron to leach into solution.
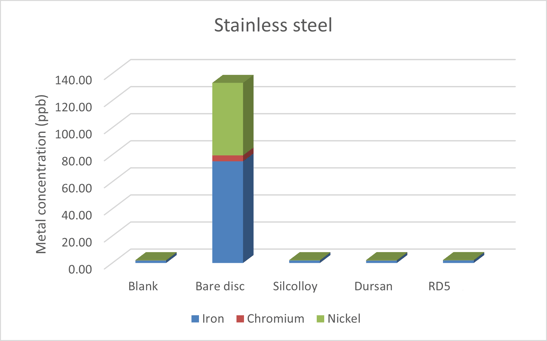
Figure 2: Stainless steel metal ion leaching into ultrapure deionized water.
|
|
Iron
|
Chromium
|
Nickel
|
|
Methanol extraction
|
812.81 ppb
|
33.55 ppb
|
61.48 ppb
|
|
DI Water extraction
|
75.13 ppb
|
4.34 ppb
|
53.62 ppb
|
Table 1: Metal ions extracted from an uncoated frit into solution after 30 days of soaking in methanol and DI water.
What remains unclear is to whether the nickel and iron metal contamination is a passivation effect or if it is truly corrosion of the material. The nickel and iron, along with their respective oxides, are much more easily extracted compared to the chromium oxide in the passive layer.3 It is possible that the contamination seen here is simply the removal of the nickel and iron from the passive layer, and no underlying metal was attacked, leaving a purer chromium oxide surface. Subsequent studies will be necessary to evaluate which mechanism is at play in this situation.

The results from the C-22 Hastelloy can be seen in Figure 3. Once again, the coated discs showed no evidence of metal ions leaching into solution. All three coatings provide sufficient protection from the DI water. Molybdenum and nickel were the major components of the metal ions in solution. It should be noted that the level of contamination was quite high compared to methanol.
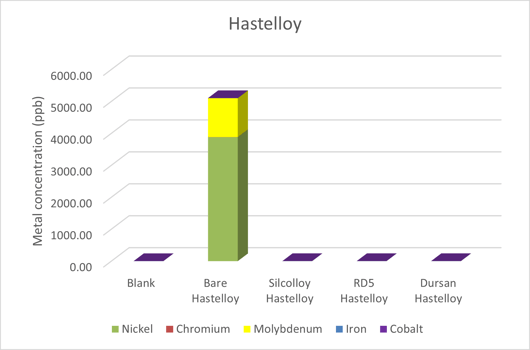
Figure 3: Metal ion contamination of ultrapure water from sintered C-22 Hastelloy discs.
Table 2 is a comparison between the methanol and water contamination. Once again, the metal ions from chromium and iron are much lower in water when compared to the methanol experiment. Counter to what was seen in the 316 stainless steel alloy, there was a dramatic increase in the corrosion of other metals in the alloy. Most notably, nickel and molybdenum leached ppm levels of metal ions into solution. C-22 Hastelloy’s oxide surface is composed largely of nickel, chromium, and molybdenum oxides. Chromium is clearly more stable in water where nickel and molybdenum suffer in water when compared to methanol. Just as with 316 Stainless Steel, it is unclear if this is a passivation effect or if this corrosion will continue with more time. Future studies are planned to determine this.
|
|
Nickel
|
Chromium
|
Molybdenum
|
Iron
|
Cobalt
|
|
Methanol
|
902.54 ppb
|
151.16 ppb
|
49.88 ppb
|
101.04 ppb
|
0.6 ppb
|
|
Water
|
3881.53 ppb
|
3.62 ppb
|
1216.41 ppb
|
1.99 ppb
|
1.32 ppb
|
Table 2: Metal ions extracted into solution after 30 days of soaking in methanol and water.
Conclusion:
UHPLC grade DI water has shown it can cause corrosion events on 316 stainless steel and C-22 Hastelloy sintered metal discs. It did not corrode titanium discs, which was expected based on previous literature. In the case of 316 stainless steel and C-22 Hastelloy, chromium and iron corrode in water at a much slower rate than was seen in methanol studies. Nickel corrosion depended on the substrate.
For 316 stainless steel, the corrosion seemed comparable to the methanol studies; however, for C-22 Hastelloy, both nickel and molybdenum experience exacerbated corrosion in the DI water. In all cases, the three coatings provided protection to the sintered metal discs from any corrosion event.
In future installments in our metal ion leaching series we'll investigate acetonitrile, which is often used as a solvent in HPLC analysis. Additionally MP35N, a nickel-cobalt-chromium-molybdenum alloy that is growing in popularity in the medical industry, will be investigated as well. Additionally, the exacerbation of nickel and molybdenum corrosion in DI water will be further investigated to determine the mechanism of corrosion.
Want to learn more about how our coatings can improve the performance of your products or process? Subscribe to our emails and blog or follow us on LinkedIn.

References:
- F. Mansfeld, J., The Effect of Water on Passivity and Pitting of Titanium in Solutions of Methanol and Hydrogen Chloride. Electrochem. Soc. 118 (1971) 1412.
- M. De Pra, G. Greco, Krajewski, M.P., Martin, M.M., George E., Bartsch N., Steiner, F., Effects of titanium contamination caused by iron-free high-performance liquid chromatography systems on peak shape and retention of drugs with chelating properties. J. Chromatogr. A. 1611 (2020) 460619.
- Menon GR, "Rough and Its Removal from High-Purity Water Systems," BioPharm 7 (1990) 40—43.
*Hastelloy® is a trademark of Haynes® International


 Porous 316 Stainless Steel and C-22 Hastelloy porous discs were purchased from Mott Corporation
Porous 316 Stainless Steel and C-22 Hastelloy porous discs were purchased from Mott Corporation





