
In part 5 of our metal ion leaching series we test HPLC grade acetonitrile. The results will surprise you!
Prevent Metal Ion Leaching Part 5: Acetonitrile Exposure
Corrosion encompasses a large variety of material degradation. It can be large enough to see visually such as the numerous examples in harsh acids or salt fog chamber experiments that SilcoTek has performed, or corrosion can be at trace levels that are not detected visually or by weight loss. We previously investigated Titanium, 316 Stainless Steel, and C-22 Hastelloy in pure methanol and DI water. Then we monitored their corrosion via ICP-MS and compared the results. Here we look at HPLC grade acetonitrile to see how it may compare to other typical solvents.
|
In this blog post you will learn:
- How various metal alloys in metal ion leaching tests.
- How SilcoTek® coatings prevent metal ion leaching and metal ion contamination.
- How acetonitrile can cause metal ion contamination in HPLC systems for some commonly used materials.
|
Background: Identifying and Preventing Metal Ion Leaching
In previous blog posts we investigated a corrosion phenomenon on common alloys for HPLC and other applications and pure solvent systems. So far, we have investigated water and methanol’s impact on stainless steel, titanium, and Hastelloy. Part one through four of this study can be found here:

In this blog, we investigate acetonitrile’s impact on all three metal alloys both with and without our coatings. HPLC systems commonly have two solvents running at a single time. One is typically aqueous (DI water) and the other is typically organic. Methanol and acetonitrile are the two most common organic solvents that are used. In previous tests we found that water was more aggressive in ion leaching with Hastelloy, and methanol was the most aggressive solvent for titanium and stainless steel. Here we show that acetonitrile is the most benign of the trio in terms of ion contamination. Never the less, aside from showing no impact to titanium (similar to water), there are still trace levels of metal ions that leach into the solution which can impact a variety of analyses.
Data and Discussion:
Porous 316 Stainless Steel and C-22 Hastelloy porous discs were purchased from Mott Corporation. Titanium porous discs were purchased from VICI precision sampling. All porous metal discs used in this study have a nominal pore size of 10 µm. One disc of each alloy type was coated with Dursan®, Silcolloy®, and a coating in development called RD5. Each coated disc and a bare disc were placed in separate polypropylene containers with 50 mL of HPLC grade acetonitrile purchased from MilliporeSigma. Additionally, one polypropylene container was filled with 50 mL of acetonitrile with no disc in it to act as a blank or control for the study. After one month of soak time, the porous disks were removed from the acetonitrile and the samples were delivered to the Energy and Environmental Sustainability Laboratories at Penn State University. Samples were then prepared by evaporating the acetonitrile and redissolving the remnants in dilute nitric acid. This was then analyzed using a Thermo Fisher iCap RQ ICP-MS.
Interested in learning more about how SilcoTek coatings can improve the performance of HPLC systems? Read our HPLC Application Guide. Click the image below to read more.

Results
Acetonitrile showed no corrosion or leaching of titanium ions into solution. All values for coated and uncoated tests were within the detection limit of the instrument (0.2 ppb) to the blank showing that no titanium ions leach into the solution. Our previous studies showed that leaching occurred in methanol (14 ppb on a bare frit) but not in water. This was supported by the literature which shows water can create a passivated oxide surface on titanium, thus not allowing corrosion to occur. Others have shown that while methanol solutions with hydrochloric acid will corrode titanium, acetonitrile solutions will not.1 They theorize that the nitrogen on the acetonitrile bonds to the titanium surface creating a passivation layer.
 The 316 stainless steel disc results can be seen in Figure 1. The coatings on the sintered metal discs block the metal ions from leaching into solution. While there was metal ion leaching into the solution, it was far less than experienced in the previous studies using methanol and water. The results look similar to the water results where iron and nickel were on the same order of magnitude and chromium leaching was much less significant, as seen in Table 1.
The 316 stainless steel disc results can be seen in Figure 1. The coatings on the sintered metal discs block the metal ions from leaching into solution. While there was metal ion leaching into the solution, it was far less than experienced in the previous studies using methanol and water. The results look similar to the water results where iron and nickel were on the same order of magnitude and chromium leaching was much less significant, as seen in Table 1.
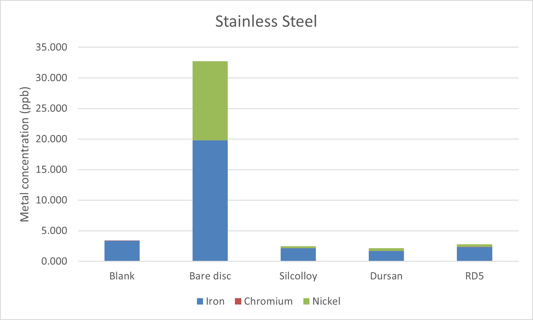 Figure 1: Stainless steel metal ion leaching into ultrapure acetonitrile.
Figure 1: Stainless steel metal ion leaching into ultrapure acetonitrile.
|
|
Iron
|
Chromium
|
Nickel
|
|
Methanol extraction
|
812.81 ppb
|
33.55 ppb
|
61.48 ppb
|
|
Water extraction
|
75.13 ppb
|
4.34 ppb
|
53.62 ppb
|
|
Acetonitrile extraction
|
19.80 ppb
|
0.02 ppb
|
12.96 ppb
|
Table 1: Metal ions extracted from an uncoated frit into solution after 30 days of soaking in methanol, water, and acetonitrile.
These results are counter to the results seen in previous studies on stainless steel with acetonitrile and methanol.2 In a study by Mowery, it was found that acetonitrile was more aggressive with stainless steel. There are some key differences between that study and this one. In their study, the acetonitrile was under high pressure and was constantly flowing. Here the acetonitrile is static and at atmospheric pressures. The high and low pressures allowed for an electrochemical difference which encourages corrosion. It is unclear what leads to the corrosion in our experiment and why it is different from theirs.
The results from the C-22 Hastelloy can be seen in Figure 2. Once again, the coated discs showed no evidence of metal ions leaching into solution. All three coatings provide sufficient protection from the acetonitrile. Nickel was the main contaminant in the acetonitrile along with a small amount of iron. Table 2 is a comparison between the methanol, water, and acetonitrile leaching tests. As was the case with stainless steel, acetonitrile is the least aggressive solvent when it comes to the leaching of metal ions into solution. Hastelloy C was investigated by Mowery, and it was shown to be comparable to stainless steel in their high pressure tests. Here, C-22 Hastelloy is by far the worst metal ion leaching substrate that was tested.
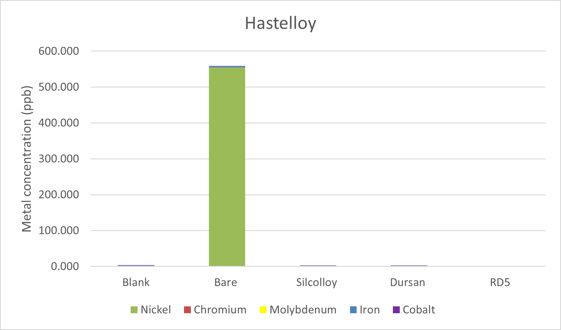 Figure 2: Metal ion contamination of ultrapure acetonitrile from sintered C-22 Hastelloy discs.
Figure 2: Metal ion contamination of ultrapure acetonitrile from sintered C-22 Hastelloy discs.
|
|
Nickel
|
Chromium
|
Molybdenum
|
Iron
|
Cobalt
|
|
Methanol
|
902.54 ppb
|
151.16 ppb
|
49.88 ppb
|
101.04 ppb
|
0.6 ppb
|
|
Water
|
3881.53 ppb
|
3.62 ppb
|
1216.41 ppb
|
1.99 ppb
|
1.32 ppb
|
|
Acetonitrile
|
554.58 ppb
|
0.21 ppb
|
0.03 ppb
|
3.81 ppb
|
0.31 ppb
|
Table 2: Metal ions extracted into solution after 30 days of soaking in methanol, water, and acetonitrile.
Conclusion:
HPLC grade acetonitrile has shown it can cause ion contamination and corrosion events on 316 stainless steel and C-22 Hastelloy sintered metal discs. Acetonitrile did not leach metal ions or corrode titanium discs. The titanium results were expected based on literature, but the stainless steel and Hastelloy results seem to contrast what is readily available in the literature. While the experiments were not identical, the inverse relationship of methanol to acetonitrile results from Mowery warrants further investigation.
The next round of experiments will investigate the three solvent’s effect on MP35N, a Nickel-Cobalt based alloy that is increasing in use for HPLC and medical components due to its bio-compatible properties.
Keep up with the latest in coating technology. Subscribe to our blog and email or follow us on LinkedIn.

References:
- Ramgopal, T. NACE CORROSION conference 2004, Paper number: NACE-04237, Published March 28, 2004.
- Mowery, R.A. “The Corrosion of 316 Stainless Steel in Process Liquid Chromatography with Acetonitrile or Methanol Carriers”. Journal of Chromatographic Science, Volume 23, Issue 1, January 1985, Pages 22-29.
*Hastelloy® is a trademark of Haynes International®



 The 316 stainless steel disc results can be seen in Figure
The 316 stainless steel disc results can be seen in Figure 


