
Can titanium leach metal ions? We discuss titanium corrosion and how to prevent it.
Can Titanium Leach Metal Ions?
Methanol corrosion is relatively slow on stainless steel substrates and was explored in a previous blog post. Here we investigate methanol’s corrosion of titanium substrates and how our Silcolloy® product can provide protection against metal ion leaching into the methanol. Dursan® and Silicon Nitride were also tested, and they did not provide protection against titanium ions leaching into solution. Possible reasons for this will be discussed.
|
In this blog post you will learn:
- How methanol can cause metal ion leaching from titanium substrates.
- How to prevent metal ion contamination for sensitive analytical and process applications.
- How silicon barrier coatings like Silcolloy and Dursan can improve corrosion resistance and prevent metal ion contamination.
|
A previous blog post discussed the issues involved with using pure organic solvent in contact with metal alloys such as stainless steel and titanium. In that study, 316 SS sintered discs were exposed to pure methanol for one month. The resulting solution was then examined via ICP-MS for metal ions contamination. The results showed that Silcolloy and Dursan were suitable coatings to protect the substrate from the methanol.
In this study titanium is investigated as a substrate for SilcoTek’s coatings. Titanium is commonly used as an alternative to stainless steel due to it being lighter, strong, and biocompatible. We find that Silcolloy performs well as a barrier, but Dursan and RD5-SiN (an experimental coating) do not provide adequate protection.
Want to learn more about how SilcoTek coatings improve corrosion resistance and prevent metal ion contamination and maintain flow path purity? Read our presentation by clicking on the presentation slide below.

Data and Discussion:
Porous grade 2 titanium discs with 10 µm nominal pore sizes were purchased from VICI Precision Sampling. They were coated with Dursan, Silcolloy, and an experimental coating we'll call RD5-SiN. After coating, the frits were placed in 50 mL of HPLC grade methanol and sealed. An uncoated disc as well as a container with no frit were also filled with methanol and sealed to act as a baseline and a blank. After one month of soak time, the porous disks were removed from the methanol and the samples were delivered to the Energy and Environmental Sustainability Laboratories at Penn State University. Samples were then prepared by evaporating 10 mL of the methanol in a PTFA vial. The remains were dissolved in mL of dilute nitric acid which was then analyzed using a Thermo Fisher iCap RQ ICP-MS.
Figure 1 shows the detected titanium from the solution of each container. The blank represents how much titanium was in the methanol bottle. The bare disc represents how much titanium is leached into the methanol without any processing at SilcoTek Corporation.
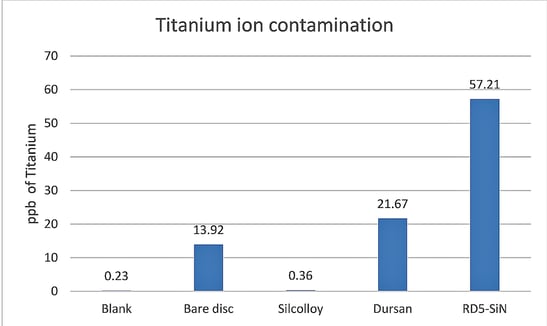
Figure 1: ICP-MS results of the methanol solution after one month of soaking.
Silcolloy® provides adequate protection to the titanium substrate from the methanol solution. It is nearly at baseline levels of titanium. The Dursan and RD5-SiN samples show significant titanium contamination of the methanol solution. This is likely due to the oxidative resistance of Silcolloy. Titanium is highly susceptible to oxidation, especially at high temperatures. Part of the Dursan and RD5-SiN processes involve high temperatures which may likely cause titanium, an element that is well known for diffusion at high temperatures, to come to the surface of the coating and become available to dissolve into the methanol during the soak. RD5-SiN is processed at a much higher temperature than Dursan. This causes a higher amount of titanium to diffuse through the coating.
As a part of this study, stainless steel discs were also tested as a follow up experiment to the previous methanol on stainless steel test. Figure 2 and 3 show the results from the previous study and this study, respectively.
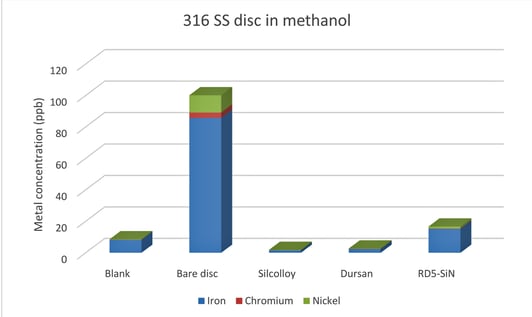
Figure 2: 316 stainless steel porous disc soaked in methanol for 1 month from TI 08-23-19.
It was noted in the previous methanol test that sample preparation may have been an issue causing contamination in some blank samples. It seemed highly unlikely that Silcolloy and Dursan would have significantly lower concentrations of metals in the solution than the blank, which was run without any disc in the solution. Upon re-examination with more care taken in sample preparation (fresh gloves between samples and more care in resealing the containers after opening), the values look more reasonable as seen in Figure 3.
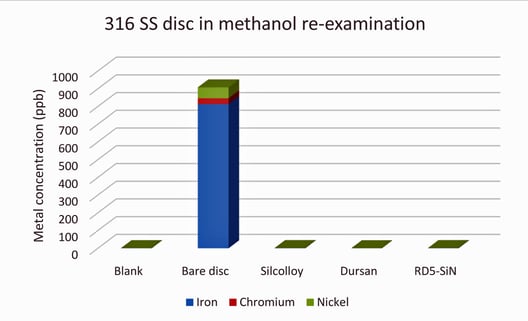
Figure 3: 316 stainless steel porous disc soaked in methanol for 1 month with more care taken in sample preparation
It is unclear as to why the bare disc in this study is an order of magnitude higher in total metal leaching into the methanol, compared to the original data illustrated in Figure 2. Some things that may affect this are things like temperature of the lab through the test, perturbation of the sealed containers during the 1-month exposure, variation in disc substrate or quality (see here for an example), or some other overlooked factor.
Due to the bare disc total metal value being so high, Figure 4 shows the blank solution as compared to the three coated discs. It should be noted that these values are very close to the detection limits of the ICP-MS for these elements (0.1-0.5 ppb) and are significantly lower than the total metal contamination from the uncoated disc, 908 ppb.
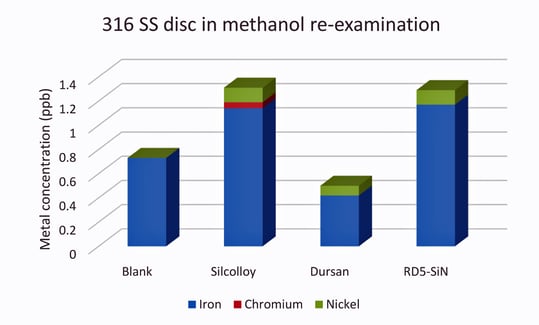
Figure 4: Stainless steel data without the bare disc for comparative purposes. The values are close enough to the blank to consider the metal ion contamination to be zero.
Conclusion:
Silcolloy provides great protection of titanium substrates from organic solvent attack of the underlying substrate. Dursan and RD5-SiN do not provide the same benefit likely caused by diffusion of the titanium into the coating which can then be dissolved easily into the methanol when soaking. All three coatings are capable of providing the necessary protection for stainless steel.
Have a question about our coatings and how we test surfaces for corrosion resistance and inertness? Contact our Technical Service Team or follow us on LinkedIn.








