
Metal ion leaching is of interest to a variety of industries. Aggressive solutions, such as acids or bleach, can cause a significant number of metal ions to dissolve into the liquid. Learn how to prevent leaching.
Ion leaching can often be seen visually. Less aggressive solvents such as methanol can cause leaching as well, but to a far lesser extent. This blog discussion investigates the ability of our coatings to prevent metal ion contamination of pure methanol from 316 stainless steel substrates. Measurements are done via inductively coupled plasma mass spectrometry (ICP-MS).
Protection Against Metal Ion Leaching by Organic Solvents
|
In this blog post you will learn:
- How to prevent metal ion leaching and contamination of 316 stainless steel when exposed to methanol
- How to improve the corrosion resistance of stainless steel.
- How to reduce contamination in HPLC systems.
|
Background:
Identifying corrosion of metal substrates is often done by visual inspection with the appearance of rust or monitored by mass changes showing that metal ions are being removed from the substrate. There are times that leaching of metal ions (aka corrosion), for example with neat organic solvents, are far less obvious, requiring a different approach to quantifying this corrosion. It would be valuable to evaluate and prevent this process, as it can have a significant impact on improving certain HPLC experiments.

The corrosion of titanium in the presence of neat methanol has been known since the 60’s when NASA found that Ti-6Al-4V (a common titanium alloy) will experience stress-corrosion cracking when exposed to methanol. Since titanium is the metal of choice for many biocompatible HPLC systems, it's critical to eliminate the exposure of the metal components to pure organic solvents. Mauro De Pra of ThermoFisher Scientific presented a talk on this subject at HPLC 2019 in Milan. He showed that titanium ions will migrate from the solvent frits to the silica packing material in the HPLC column. Once there, the titanium ions will react and bind with silanols on the surface of the silica. These immobile ions can cause issues with certain compounds which chelate to them. One such compound is ciprofloxacin, a common antibiotic, which will show significant tailing and peak distortion if run through a titanium contaminated column.
This issue is not limited to titanium. Mel Euerby is currently a scientist with Shimadzu, and he wrote a paper in 1995 while he worked for Fisons Pharmaceuticals (now a part of AstraZeneca) that showed a similar issue can occur with stainless steel. He showed that both acetonitrile and methanol could cause iron ions to leach into the silica bed causing poor peak shapes when running a separation with a metal chelating compound, 2,3-dihydroxynapthalene. He also showed that soaking frits in methanol can extract up to 1µg of total iron, which is not measurable via visual changes nor via mass change with a standard lab balance.
SilcoTek has previously shown that the Dursan coating process can assist with any metal chelating compound separation by providing a barrier layer between the stainless steel substrate and the analytical flow path. This blog post will investigate whether Dursan and other SilcoTek coatings can prevent metal ions from leaching into pure organic solvents.
Data and Discussion:
Porous metal discs with 10 µm nominal pore sizes were purchased from McMaster Carr. Coatings to be tested were Dursan, and Silcolloy. Once the coatings were complete, the coated frits and an uncoated frit were placed individually in polypropylene plastic containers which were filled with 100 mL of HPLC grade methanol and closed. One plastic container was filled with 100 mL of HPLC grade methanol and closed without a sintered disc present to act as a baseline for the experiment. After an exposure time of one month, the sintered discs were removed from the methanol and the samples were delivered to the Energy and Environmental Sustainability Laboratories at Penn State University. Samples were then prepared by evaporating 10 mL of the methanol in a PTFA vial. The remains were dissolved in approximately 5 mL of dilute nitric acid which was then analyzed using a Thermo Fisher iCap RQ ICP-MS. Iron, nickel, and chromium were measured and the results can be found in Table 1.
Table 1: ICP-MS results of sintered discs soaked in methanol for one month. All values are in PPB.
|
|
Iron
|
Chromium
|
Nickel
|
|
Blank (no disc)
|
8.22
|
0.11
|
0.26
|
|
Bare disc
|
85.7
|
3.44
|
11.0
|
|
Dursan
|
2.34
|
0.14
|
0.23
|
|
Silcolloy
|
1.55
|
0.07
|
0.22
|
The uncoated sintered disc showed a significant increase in the amount of metal ions that have leached into the solution. It should be noted that the metal content in the Dursan and Silcolloy coated discs appear to be lower than the blank. These values may seem odd since it appears that Dursan and Silcolloy have an adsorptive property to them when it comes to some metal ions. Nothing in our previous testing indicates this to be true.
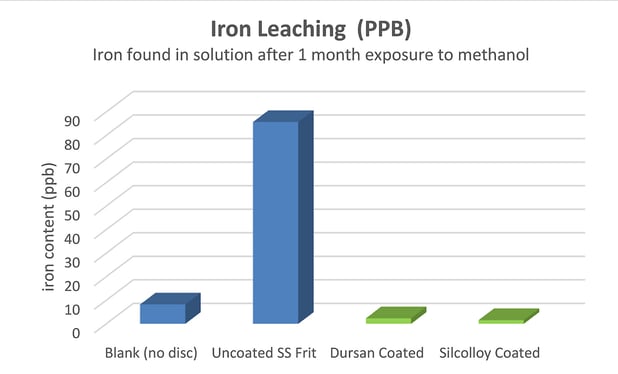
It is important to keep in mind two things when considering these numbers. First, the sample preparation process was not perfect. Used glassware, a non-cleanroom environment, and sample transfer could all lead to minor variances in metal ion contaminants. Second, the level of metal contamination is low enough that sample to sample variation is just noise in the experiment. All coatings are close enough to the blank’s level of metal ions to say that the coating was successful in blocking metal ions from leaching into the solution. A bare stainless steel disc created at least an order of magnitude greater metal signal in all three elements of interest after the one month immersion time in methanol.
Conclusion:
Over the course of a one-month immersion in pure methanol, a stainless steel sintered disc contaminates the methanol with about 80 ppb of iron, 3 ppb of chromium, and 10 ppb of nickel. These values are only semi-quantitative as sample preparation may lead to minor contaminations. Applying a CVD coating to the metal samples creates a sufficient barrier to stop the metal ion leaching into the solvent. Since methanol causes ion leaching to a greater extent with titanium substrates, it is worthwhile to repeat this experiment with a titanium substrate rather than stainless steel. These results also show that repeating Mel Euerby’s experiments will be of interest to the HPLC community. The ability to maintain the mechanical robustness of metal components, but have a metal-free analytical flow path is a game changing technology for the liquid chromatography community.
Have a question about corrosion or surface inertness? Ask our experts or follow us on LinkedIn.





