
In this blog post we investigate the corrosion of C-22 and 316L stainless steel in pure methanol and the impact that SilcoTek’s coating, Silcolloy, can have on improving corrosion resistance and preventing metal ion leaching.
Preventing Hastelloy Metal Ion Leaching
Corrosion can impact materials and processes in several ways. It can be severe enough to see visually and damage components (as seen in the numerous test examples in harsh acids or salt fog chamber experiments that SilcoTek® has performed). Or corrosion can be found at trace levels that are not detected visually or by weight loss, but can severely contaminate processes and products.
We previously investigated both Titanium and 316 Stainless Steel in pure methanol and monitored their corrosion via ICP-MS. Exotic alloys, such as Hastelloy, are also used in harsh corrosion conditions. In this blog post we investigate the corrosion of C-22 and 316L stainless steel in pure methanol and the impact that SilcoTek’s coating, Silcolloy®, can have on corrosion and contamination.
|
In this blog post you will learn:
- How methanol can leach metal ions from Hastelloy C-22.
- How methanol can leach metal ions from 316L stainless steel.
- How SilcoTek coatings such as Silcolloy can virtually eliminate metal ion leaching when exposed to methanol.
- Get comparative data on metal ion leaching rates for Hastelloy, 316L stainless steel, and Silcotek coatings.
|
In a previous blog post we discussed the issues involved with using pure organic solvent in contact with metal alloys such as stainless steel and titanium. In that study, 316 SS and titanium sintered discs were exposed to pure methanol for one month. The resulting solution was then examined via ICP-MS for metal ion contamination. The results showed that Silcolloy® and Dursan®, were suitable coatings to protect stainless steel from the methanol attack, while only Silcolloy effectively stopped titanium ions from being leached into solution. In this blog post Hastelloy (specifically C-22) is similarly investigated as a substrate for SilcoTek’s Silcolloy® coating.
Background
Hastelloy® alloys were developed for severe corrosion conditions. These high nickel exotic alloys typically work well to battle halide and other acid corrosion. They also have great resistance to oxidation which make them an appealing substrate for semiconductor, aerospace, and oil and natural gas industries. For reference, Table 1 shows the composition of C-22 Hastelloy from Haynes® International, the producer of Hastelloy materials.*
| Element |
Weight % |
| Nickel |
56% (balance) |
| Chromium |
22% |
| Molybdenum |
13% |
| Iron |
3% |
| Tungsten |
3% |
| Cobalt |
2.5% max |
| Copper |
0.5% max |
| Manganese |
0.5% max |
| Vanadium |
0.35% max |
| Silicon |
0.08% max |
| Carbon |
0.01% max |
Table 1: Composition of C-22 Hastelloy® according to Haynes® International.
There is a wealth of literature supporting the use of Hastelloy and 316L stainless steel for visual or severe corrosion conditions, but when it comes to trace levels of corrosion, very little is known. Immersion testing conducted by SilcoTek Research Staff found that bare uncoated stainless steel and C-22 sintered discs when exposed to pure methanol can leach over 1 ppm total metal into solution, where the same discs coated with Silcolloy showed no measurable loss of metal ions, indicating only baseline levels of leaching relative to experimental controls.
Methanol Leaching Data and Discussion. Improved Protection Against Metal Ion Contamination

Porous C-22 Hastelloy and 316L stainless steel discs with 10 µm nominal pore sizes were purchased from Mott Corporation. They were coated with Silcolloy silicon barrier coating. After coating, the discs were placed in 50 mL of HPLC grade methanol and sealed. Uncoated discs as well as a container with no disc (a solvent blank test control to establish baseline response) were also filled with methanol and sealed. After one month of soak time, the porous disks were removed from the methanol and the samples were delivered to the Energy and Environmental Sustainability Laboratories at Penn State University. Samples were then prepared by evaporating 10 mL of the methanol in a PTFA vial. The remains were dissolved in 5 mL of dilute nitric acid which was then analyzed using a Thermo Fisher iCap RQ ICP-MS.
Figure 1 shows the total metal ion concentrations from the solution of each container. The blank represents the level of metal ions in the methanol bottle, leached from the sealed container, sample preparation, or absorbed via environmental sources. The bare Hastelloy and stainless steel discs represent the level of metal ions leached into the methanol without any coating protection provided by processing at SilcoTek Corporation. The level of metal ion contamination for the uncoated discs was significant, with the Hastelloy discs exceeding 1200 ppb contamination.
The coated discs showed little to no appreciable level of contamination.
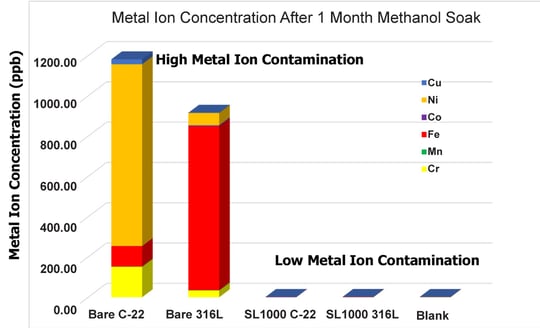
Figure 1: Metal ion concentrations in methanol. The blank is methanol from the bottle with no disc. The four others are an uncoated Hastelloy disc, an uncoated stainless steel disc, and two Silcolloy® coated discs soaked in methanol for 1 month at room temperature. The coated discs showed minimal contamination.
Table 2 (below) shows the raw values from the Hastelloy sample along with the total metal ion content. The SilcoTek coated Hastelloy disc reduced metal ion contamination by 700x or more.
|
Sample
|
Ni (ppb)
|
Cr (ppb)
|
Mo (ppb)
|
Fe (ppb)
|
W (ppb)
|
Co (ppb)
|
Cu (ppb)
|
Mn (ppb)
|
V (ppb)
|
Total metal content (ppb)
|
|
Blank (methanol only)
|
0.5
|
0.01
|
0.03
|
0.66
|
0.05
|
0.00
|
0.14
|
0.14
|
0.01
|
1.54
|
|
Uncoated Hastelloy Disc
|
902.54
|
151.16
|
49.88
|
101.04
|
3.46
|
0.60
|
24.53
|
1.36
|
1.00
|
1235.57
|
|
Silcolloy Coated Hastelloy
|
0.15
|
0.02
|
0.05
|
0.83
|
0.11
|
0.01
|
0.15
|
0.16
|
0.18
|
1.66
|
Table 2: Metal content in methanol measured via ICP-MS. All units are ppb.
Due to the bare disc total metal ion value being so high and to improve the comparative resolution of data results, Figure 2 shows the blank solution as compared to the three coated discs. It should be noted that these values are very close to the detection limits of the ICP-MS for these elements (0.1-0.5 ppb) and are significantly lower than the total metal contamination from the uncoated disc, at 1235 ppb contamination.
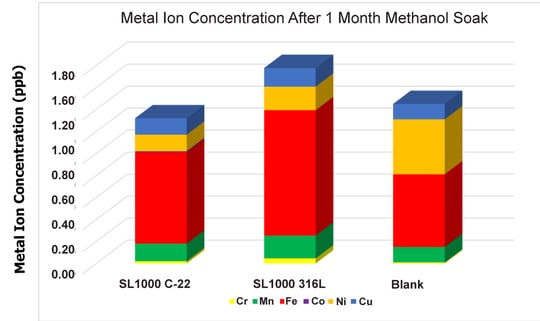
Figure 2: This is data transposed from Figure 1, but with the bare uncoated discs removed for better resolution of the coated discs.
Conclusion:
Silcolloy® provided sufficient protection against metal ion leaching into the methanol during a one-month exposure at room temperature. It should be noted that while these sintered discs are often used for filtering solvents and will experience solvent flow, this experiment was done under static conditions.
The metal ionic states as well as the solubility limits of those metal ions in methanol are not readily available through our experiments nor in the literature. It is possible that utilizing these uncoated porous discs for their intended purpose will result in larger quantities of metal ions being leached into flow paths as they provide a constant supply of uncontaminated solvent and are in flow conditions rather than static.
Have a question about how our coatings can prevent product contamination and improve the performance of your process or products? Just send us a chat or contact our Technical Service Team. You can also follow us on LinkedIn to read about the latest in high performance coatings.

* Hastelloy® and Haynes® are trademarks of Haynes International Inc.





