
Ionic contamination in HPLC systems can result in peak distortion, split peaks, and lost peaks. Left unchecked, severe ion contamination can reduce column life & damage the column resin bed; leading to hours of troubleshooting and system cleaning.
Removing metal ions can entail flushing the system with a chelating agent or rinsing the system with an acid wash which of course can lead to more corrosion of metal surfaces and yes more ionic contamination. Maddening to say the least!
 Sources of ionic contamination range from stainless steel frits, stainless steel LC pumps, metal transfer tubing and any stainless steel found in the sampling and transfer system flow path. The overall cost of metal ion contamination can be as little as a few thousand dollars invested in troubleshooting and column or component replacement, to many thousands in lost productivity and lost orders.
Sources of ionic contamination range from stainless steel frits, stainless steel LC pumps, metal transfer tubing and any stainless steel found in the sampling and transfer system flow path. The overall cost of metal ion contamination can be as little as a few thousand dollars invested in troubleshooting and column or component replacement, to many thousands in lost productivity and lost orders.
That's why it pays to avoid the whole crisis situation whenever possible by investing in system flowpaths that eliminate the analyte metal interface and incorporate both surface chemical inertness and corrosion resistance.

Prevent Corrosion With HPLC Coating
SilcoTek® barrier coatings like Dursan® and Dursox® prevent corrosive attack and ion leaching from stainless steel and alloy surfaces; improving overall system purity and corrosion resistance. Controlled acid immersion studies demonstrate a significant reduction in corrosive attack when stainless steel surfaces are coated with an inert corrosion resistant silicon surface like Dursan.
Immersion test results:
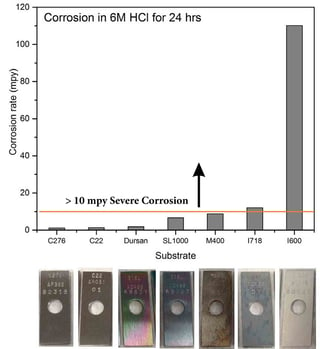
After exposure to concentrated HCl for 24 hours, the Dursan coated coupon demonstrates super alloy level corrosion resistance, with very low metal loss.
Looking at the acid tells another story. After exposure, even super duplex steel contaminates the acid; turning the hydrochloric acid green. Major ion contamination!

The Dursan® container remains clear after exposure. No ion contamination!

Make the surface inert
The less interaction with potentially reactive metal surfaces the better. Especially when testing for ultra low detection limits. A corrosion resistant surface is not necessarily inert nor is an inert surface guaranteed to be corrosion resistant. Surfaces should be tested for both corrosion and reactivity to be sure you're getting a high performance flowpath.
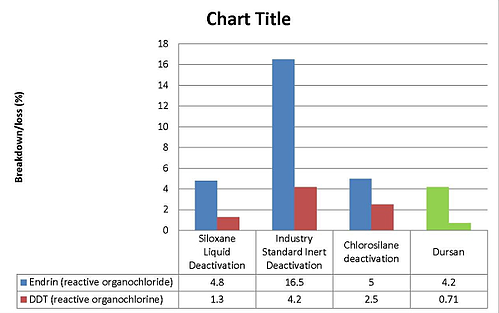
Comparative testing shows a silicon surface applied by chemical vapor deposition will improve both corrosion resistance and inertness in HPLC flowpaths. Comparing Dursan to popular deactivation chemistries shows comparable inertness to reactive compounds.
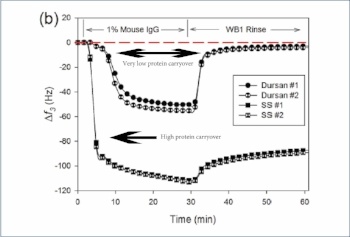
An inert surface will prevent contamination and carryover. Quartz crystal micro balance (QMCD) analysis of stainless steel and Dursan surfaces show significant binding and carryover of protein molecules on stainless steel while the Dursan surface does not allow protein binding after rinse.
If you want to learn more about how our coatings can improve HPLC system performance, watch our webinar.


 Sources of ionic contamination range from stainless steel frits, stainless steel LC pumps, metal transfer tubing and any stainless steel found in the sampling and transfer system flow path. The overall cost of metal ion contamination can be as little as a few thousand dollars invested in troubleshooting and column or component replacement, to many thousands in lost productivity and lost orders.
Sources of ionic contamination range from stainless steel frits, stainless steel LC pumps, metal transfer tubing and any stainless steel found in the sampling and transfer system flow path. The overall cost of metal ion contamination can be as little as a few thousand dollars invested in troubleshooting and column or component replacement, to many thousands in lost productivity and lost orders. 






