We discuss how metal contamination can effect food quality, oxidation, and proteins. Learn how silicon barrier coatings prevent metal contamination.
We discuss how metal contamination can effect food quality, oxidation, and proteins. Learn how silicon barrier coatings prevent metal contamination.
The Impact of Metal Contamination on Food Quality
Metals and metal alloys are a fantastic material option for food processing equipment and for a variety of other industrial processes. Alloys like stainless steel are strong, robust, and easily machined, especially when compared to plastics or ceramics. One issue found with most metals when exposed to corrosive environments is varying degrees of rust, corrosion, and metal leaching in the product flow path. We discuss ways coatings, like Silcolloy®, prevent metal contamination and improve food quality.
|
In this blog post you will learn:
- How metal ion contamination can impact food quality.
- How silicon coatings act as a barrier to prevent metal ion leaching.
- How various materials like stainless steel perform when exposed to DI water, methanol, and other chemicals.
|
The Degrees of Corrosion in Metal
Figure 1 shows the varying degrees of metals interacting with their chemical environments. Obvious corrosion and rouging are signs of metal ion introduction into the product. Typically, obvious corrosion leads to structural concerns and higher maintenance cost as well. Both examples are visual; however, alloys can also leach trace levels of metal ions into solution. This is often unnoticed and/or overlooked by many users of sophisticated alloys such as stainless steels and specialty alloys. It is certainly of interest to many in the biopharmaceutical industry as the metal ions can cause an increase in impurities and degrade the end product more quickly.
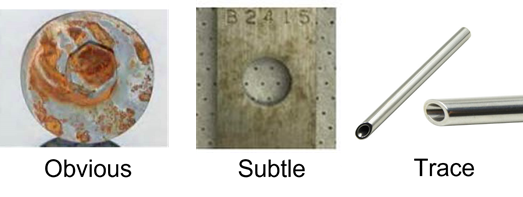
Figure 1: Three examples of metal materials reacting with their environment. Each will have differing levels of concern to different industries, experiments, and individuals.
While this problem is currently not largely considered by those in the food and beverage industry, there are areas where it should be given consideration. It should be highlighted that metals exposure/contamination can be split into two categories according to the FDA.
There are metals that the human body requires as a part of a recommended daily intake. These include iron, chromium, manganese, and molybdenum. These metals can also be found in stainless steel.
The second category, toxic metals that are harmful to health, are generally avoided in food service applications but these metals may be present in alloy form. For example, the only metal found in stainless steel that the FDA suggests is harmful to health is nickel, which if leached into a food or beverage may impact food quality or result in higher consumption of that metal. This blog post will not focus on the health issues associated with consuming metal ions in food and beverage industry, but rather the impact that metal ions have on the end product and processing of foodstuffs.

Table 1, below, briefly highlights a few examples where metal ion contamination can cause issues. Most of the examples relate to the oxidative properties of metal ions, and their ability to react with proteins and lipids. This is not an exhaustive list, however, The book “Oxidation in foods and beverages and antioxidant applications” is recommended reading for more information on oxidation in foods.
Table 1: Various issues that metal ion contamination could have on foodstuffs.
|
Foodstuff
|
Issues that metal ions can cause
|
|
Baby formula
|
Formula contains numerous lipids (fats) that are prone to oxidation from iron or copper ions. Lactoferrin (a metal chelating protein) has been used to sequester the iron and limit the lipid oxidation.1
|
|
Fish Oil
|
Omega-3 fatty acids are prone to oxidation by excess iron in the oil product. Apotransferrin, phytic acid, and EDTA were shown to inhibit lipid oxidation in a salmon oil-in-water emulsion.2
|
|
Milk and Mayonnaise
|
Lipid breakdown was caused by iron metal ions. Lactoferrin, phytic acid, and EDTA were shown to be good preservatives by binding to the iron in solution.3
|
|
Cooked beef patties
|
Potato proteins were showed to slow the break down of beef proteins and fats when stored in a refrigerated location. The copper and iron was able to selectively bind to the potato protein in the patties and not allow them to attack the fats and beef proteins.4
|
|
Wire
|
Transition metal oxidation causes accelerated aging in wines. Often sulfites are added to decrease the rate at which these reactions take place. Many people have allergic reactions to sulfites which make sulfites an undesirable additive if unneeded.5
|
The metals present in these foods are likely introduced by some combination of the endogenous metal content of the raw materials to make the product and metals released from the processing equipment. The contribution from each source will vary depending on the product and the processing involved.
The solution for many of these oxidation related problems involve either the removal of metals and metal ions by means of binding and filtering, or the addition of a variety of preservatives that act as anti-oxidants in order to limit the damage the metal ions can do to the product. Both of these techniques have shown to have unintended consequences in either sensory differences or regulatory issues, as certain metal chelating preservatives can have health impacts.
If we focus on the processing equipment we find that preventing the interaction of food products with the flow path surface, commonly stainless steel, can go a long way to preventing the release of metal ions into the food product, potentially avoiding added binding, filtering or preservatives.
Learn how SilcoTek® coatings can improve products in food flavor and fragrance applications.

Preventing Metal Ion Contamination
 Metal ion leaching is of interest to a variety of industries. Chemical exposure to solutions, such as, organic solvents, deionized water, acids or bleach, can cause a significant amount of metal ions to dissolve into the liquid, causing product contamination, system corrosion, and test problems in analytical and high purity processes including:
Metal ion leaching is of interest to a variety of industries. Chemical exposure to solutions, such as, organic solvents, deionized water, acids or bleach, can cause a significant amount of metal ions to dissolve into the liquid, causing product contamination, system corrosion, and test problems in analytical and high purity processes including:
- Beer, wine and other beverage production
- Food Processing
- Biopharmaceutical production
- Precision cleaning applications
- HPLC analytical columns and flow paths
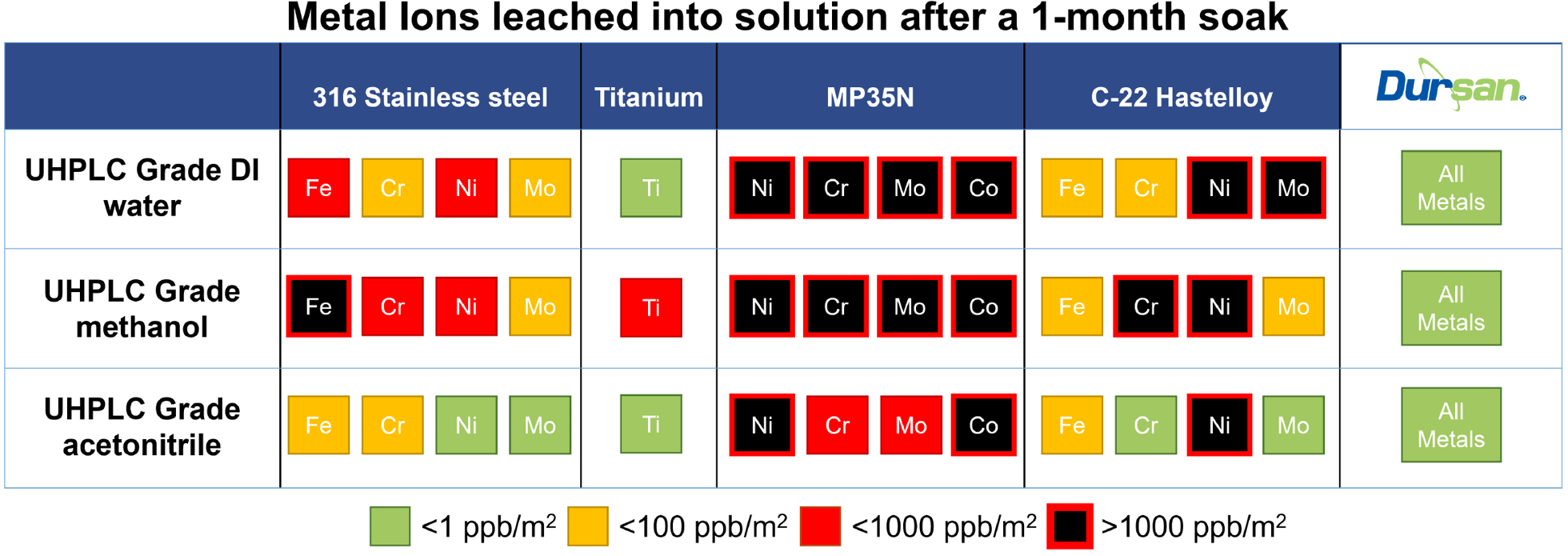
SilcoTek® offers CVD coating solutions to make your process, analytical, and sampling systems perform better. Our chemically inert coatings improve test accuracy and reliability by preventing contamination from metal ion leaching caused by chemical reactions between the sample and flow path surface. The summary below demonstrates how metal ions can be readily leached into flow path solutions and how coated surfaces (right column, green) prevent contamination under all conditions tested.
SilcoTek's CVD silicon coating process bonds inert, non-reactive coatings, like Dursan®, Silcolloy®, and SilcoNert®, to the surface. The chemically inert coatings prevent interaction of the sample with the reactive flow path surface.
SilcoTek has conducted several studies relating to the effectiveness of our CVD coatings in preventing metal ion leaching from various surfaces when exposed to organic solvents and deionized water. Read the Technical Insights below to further understand how SilcoTek coatings improve product and test quality.
Metal Contamination in Deionized Water Exposure
In this study we investigate DI water’s impact on titanium, Hastelloy, and stainless steel. Among numerous other uses, DI water is commonly used as a solvent for HPLC analysis, in food and drug production, in high purity ultrasonic cleaning, and as a precursor in precision manufacturing like semiconductor manufacturing applications.

Results of the tests show that titanium alloys do not suffer metal ion leaching in DI water, while both stainless steel and Hastelloy show large amounts of metal ion leaching, specifically an abnormally large amount of nickel leached into DI water when compared to the methanol leaching studies.
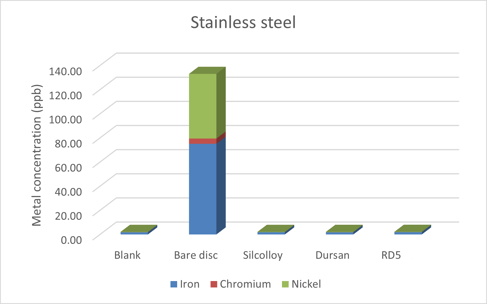
Metal Contamination From Titanium Exposure
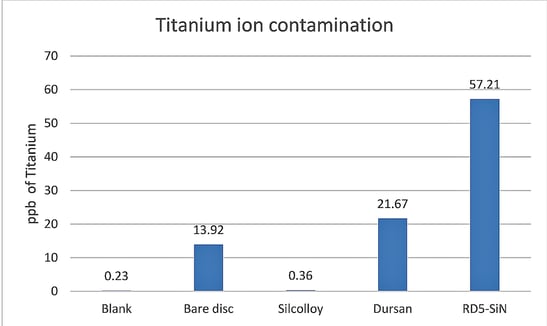
In this study titanium is investigated as a substrate for SilcoTek’s coatings. Titanium is commonly used as an alternative to stainless steel due to it being lighter, strong, and biocompatible. We find that Silcolloy performs well as a barrier, but Dursan and a test coating,RD5, do not provide adequate protection.

The Silcolloy coated titanium sample improved the resistance to ion contamination in methanol.
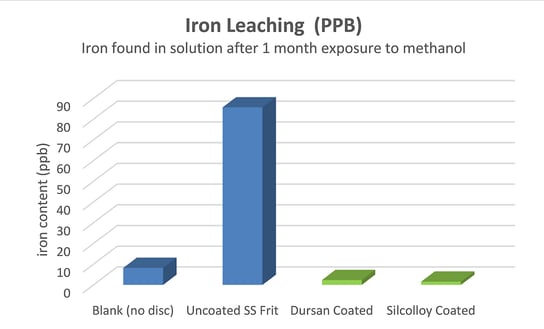
Metal Contamination Resulting From Organic Solvent Exposure
This Technical Insight investigated whether Dursan and other SilcoTek coatings can prevent metal ions from leaching into pure organic solvents.

The study showed that high purity organic solvents like methanol can cause contamination and corrosion in stainless steel flow paths. Coated stainless steel surfaces prevent ion leaching, corrosion, and contamination of analytical test and process streams.
SilcoTek coatings can be applied to most critical flow paths found in analytical and food processing applications. To read more about how our coatings can reduce or eliminate metal contamination, go to our Metal Contamination Page.
References:
- Satue-Gracia, M. T., Frankel, E. N., Rangavajhyala, N., and German, J. B. (2000). Lactoferrin in infant formulas: Effect on oxidation. J. Agric. Food Chem., 48, 4984–4990.
- Mancuso, J. R., McClements, D. J., and Decker, E. A. (1999). The effects of surfactant type, pH, and chelators on the oxidation of salmon oil-in-water emulsions. J. Agric. Food Chem., 47, 4112–4116.
- Nielsen, N. S., Petersen, A., Meyer, A. S., Timm-Heinrich, M., and Jacobsen, C. (2004). Effects of lactoferrin, phytic acid and EDTA on oxidation in two food emulsions enriched with long-chain polyunsaturated fatty acids. J. Agric. Food Chem., 52, 7690–7699.
- Wang, L. L., and Xiong, X. L. (2005). Inhibition of lipid oxidation in cook beef patties by hydrolyzed potato protein is related to its reducing and radical scavenging ability. J. Agric. Food Chem., 53, 9186–9192.
- Elias, R.J., and Waterhouse, A.L. (2010). Controlling the Fenton Reaction in Wine. J. Agric. Food Chem., 58, 1699-1707.
 We discuss how metal contamination can effect food quality, oxidation, and proteins. Learn how silicon barrier coatings prevent metal contamination.
We discuss how metal contamination can effect food quality, oxidation, and proteins. Learn how silicon barrier coatings prevent metal contamination.