
Corrosion can damage process components and reduce efficiency, but metal ion contamination can ruin a product, your process and your business. We discuss how to improve corrosion resistance and prevent leaching.
Stop Metal Ion Leaching in Semiconductor and Analytical Applications
SilcoTek coatings are a go-to solution for solving contamination problems from metal ion leaching in etch tools, including gas delivery lines, chambers, and exhaust systems. Metal ions like iron (Fe) leach out of stainless steel processing equipment as a result of corrosive mechanisms and aggressive process chemistries. SilcoTek coatings provide a high purity silicon barrier to prevent this contamination and inhibit corrosion. This can be especially vital when labs or processes need test for trace chemicals in ppm concentrations or to achieve ppq performance in the lab.
Hyper-expensive super alloy materials like Hastelloy® have been used to solve corrosion problems, but the costs and difficulty in machining/logistics are a major headache to analytical and semiconductor customers. Ultimately these alloys can also create ion contamination problems as well. That's why bonding an inert silicon coating to the base metal to prevent process fluid exposure to metal surfaces is a key step to improving test or process purity.
Have a question about this blog or corrosion resistance? Click the box below to discuss your application with one of our coating scientists.
Have a Question? Contact Our Technical Service Team
In this blog post we'll discuss metal ion contamination and how to improve flow path corrosion resistance.
|
In this blog post you will learn:
- How Silicon coatings improve flow path corrosion resistance
- How Coatings like Dursan, Silcolloy, and Dursox reduce metal ion leaching and metal ion contamination
- How to consult with our team to find the best corrosion solution for your application.
|
Do silicon coatings contain metals that can leach into my process?
The benefit of a barrier coating is that it prevents interaction of the process fluids or test analyte with reactive and potentially ion contamination prone surfaces like stainless steel or super alloys. But if the coating itself contains metals the benefit is lost. Even trace metals can be a major problem in trace analysis or when using in sensitive manufacturing environments like semiconductor etch processes. The XPS analysis of our Dursox® coating shows that the silicon coating does not contain trace metals and is 99.98% pure.
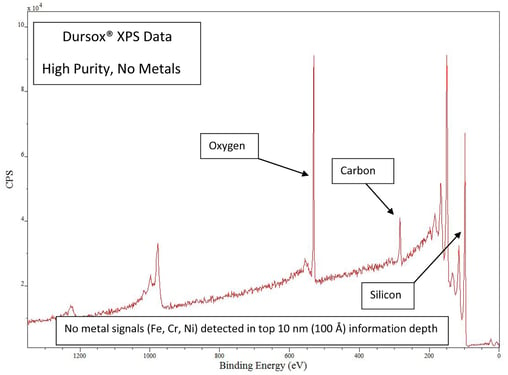
Will silicon coatings react in corrosive environments?
Corrosives like HBr can corrode stainless steel and cause subsequent iron contamination in the product flow path. Coatings like Silcolloy® significantly reduce corrosion. Learn more about how our coatings improve corrosion resistance. Read our latest corrosion presentation.

The graph below shows a large reduction in hydrogen bromide corrosion after exposure for 24 hours. The Silcolloy coated test coupon remains nearly corrosion free while the stainless steel coupon is severely corroded.
/ASTM%20G31%20Silcolloy%20%206N%20HBr.png?width=425&name=ASTM%20G31%20Silcolloy%20%206N%20HBr.png)
Even under elevated temperatures, the corrosion rate is reduced by orders of magnitude. Uncoated 316 stainless steel exposure to hydrochloric acid under extreme temperatures results in excessive corrosion (graph below). The silicon coated 316 stainless steel coupon reduced corrosion by 97%.
/hcl%20elevated%20temp.png?width=480&name=hcl%20elevated%20temp.png)
Bleach is used to remove bio contamination from stainless steel surfaces, but it can also corrode stainless steel and cause metal ion contamination in process fluids. Dursan and Silcolloy coated flow paths are protected from excessive corrosion, preventing contamination while extending the life of process equipment and components.
/Bleach%20ss%20vs%20dursan.jpg?width=375&name=Bleach%20ss%20vs%20dursan.jpg)
Benefits of coated surfaces? Increased up-time, less maintenance.
We tested equipment up-time (measured in RF Hours, below) and found that silicon coated stainless steel outperformed uncoated stainless steel by more than 6x.
/Equipment%20Uptime%20HBr%2072%20hours.png?width=437&name=Equipment%20Uptime%20HBr%2072%20hours.png)
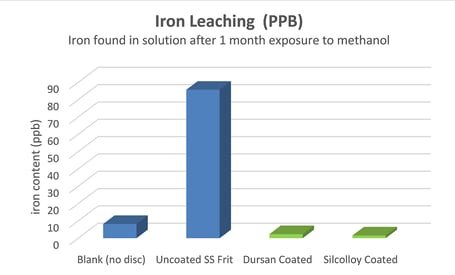
What about iron leaching? Can corrosives leach iron through the coating?
After a month of exposure to methanol, stainless steel frits contaminated the methanol with iron. Silcolloy and Dursan coated frits did not leach iron. Even extreme surface area components like sintered metal frits are protected from process contamination.
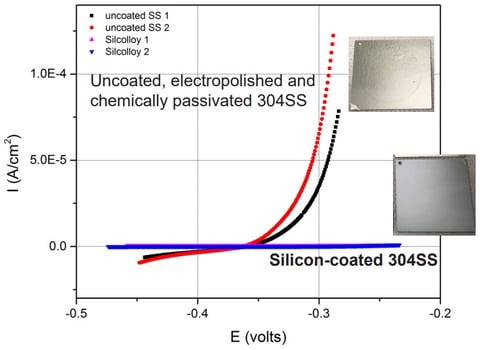
Polarization scan testing (below) shows the silicon coated 304 stainless steel coupon resists pin-holing and the potential for metal ion leaching. Assuring processes and analytical test streams remain free of ion contamination. The silicon coated stainless steel coupon (blue line) shows no change in electrochemical current throughout the voltage range while the uncoated stainless steel coupon shows a significant change increase in pitting potential.
 Have a question about how to improve corrosion resistance and stop metal ion contamination? Contact our Technical Service Team, or join us on LinkedIn.
Have a question about how to improve corrosion resistance and stop metal ion contamination? Contact our Technical Service Team, or join us on LinkedIn.




/ASTM%20G31%20Silcolloy%20%206N%20HBr.png?width=425&name=ASTM%20G31%20Silcolloy%20%206N%20HBr.png)
/hcl%20elevated%20temp.png?width=480&name=hcl%20elevated%20temp.png)
/Bleach%20ss%20vs%20dursan.jpg?width=375&name=Bleach%20ss%20vs%20dursan.jpg)
/Equipment%20Uptime%20HBr%2072%20hours.png?width=437&name=Equipment%20Uptime%20HBr%2072%20hours.png)


