
In last week's blog we discussed what is a CVD coating. This week let's discuss why we specify a CVD coating thickness range rather than a specific thickness. The short answer, we probably could but you wouldn't want to pay for it!
First a quick review of the chemical vapor deposition (CVD) coating process to refresh your memory about our process. If you're already aware of how CVD works than as Monty Python would say, "skip a bit".
|
In this blog post you will learn:
- The sources of coating thickness variation in silicon CVD processes.
- How the part surface can impact coating thickness variation.
- How the coating reaction rate can impact thickness.
- What tools are used to characterize a surface for optimum coating quality.
|
In last week's blog we discussed what CVD coatings are and how they are applied. We stated that chemical vapor deposition (CVD) is a process that involves the reaction of a volatile precursor which is injected into a chamber (typically under vacuum). The chamber is heated to a reaction temperature that causes the precursor gas to react or break down into the desired coating and bond to the material surface. Over time the coating material builds on the surface and creates a coating throughout the exposed part's surface. Want to learn more about our coating process? Go to our Coating Technology and Quality page.
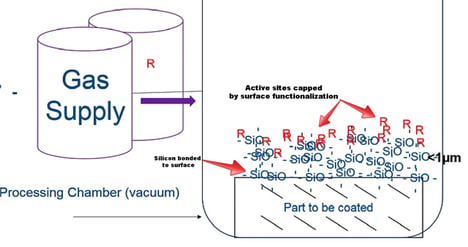
The silicon will bond to any exposed surfaces (both internal and external) while the chlorine and hydrochloric acid gas will be vented from the chamber and scrubbed according to appropriate regulatory requirements. The CVD coating system may look something like the diagram above. In this example the desired coating material is a silicon oxide base coating with a functionalized (R) surface which is intended to cap any remaining active sites on the coating surface. This makes for a superior non reactive surface.
This chemical vapor deposition differs from physical vapor deposition (PVD) which involves vaporizing a coating material and spraying or condensing the vaporized material on the surface. This results in a line of sight application compared to chemical vapor deposition which is non line of sight. Comparing coated parts would look something like the graphic below.
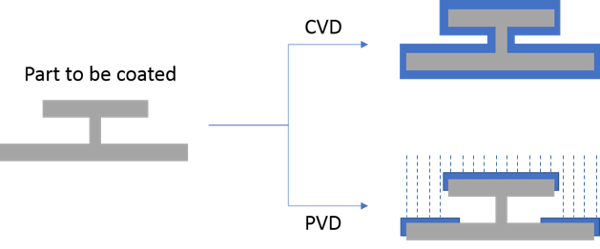
I like to think of PVD part coverage being something like snow covering my deck furniture. Under my table, there's little to no snow while there's lots of snow on top of the table (Yes I know I should have put the furniture in the garage months ago!)*. With PVD, surfaces must be exposed to the application vapor; shielded areas won't be coated (like under my table).
The chemical vapor deposition will react and apply to any area the coating gas can get into. The deposition process allows us to build a silicon layer on the surface one Angstrom (0.1 nanometer) at a time. In theory we should be able to specify a coating to the nanometer, so why do CVD coatings have a thickness range? In practice, variation and process limitations result in specifying a range of coating thickness.
Sources of variation in the coating process
The table below summarizes the range of coating thickness for each coating. So why the difference in thickness? Well there are lots of contributing factors that add up to variation in the thickness of our coatings.
To to our Coating Specifications and Properties page to get complete coating specs.

Here are a few contributing factors that add to variation in CVD coating thickness and thickness specifications.
1. Part surface
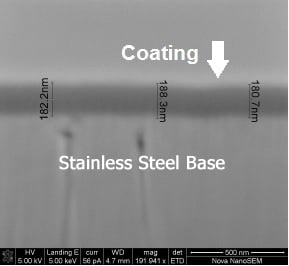
Is the part electropolished to a mirror finish with little surface roughness (5 ra for example?) or is the part cast with an extremely rough surface? Getting back to that snow analogy, think of a part surface like a mountain range. When snow falls on the mountains it covers the peaks and valleys but can cover more heavily in the valleys compared to a shear cliff wall for example. Our coating process will coat the entire part but may preferentially coat some areas of a rough surface like a peak. We're not talking about a large amount of variation but the surface is a contributor. The SEM image below is a good example. You can see some changes in the part surface (light gray) and the impact on the overall coating thickness. Over the small sample area of the surface you can see 8nm in thickness variation.
2. Part configuration
As noted, our process will coat the interior of a part as well as the exterior. Here the available coating volume can play a part in coating thickness. A small confined area, like the interior bore of a needle, will tend to have slightly less coating available to bond to the surface. We have a few tricks that we use to minimize the effect but in some part configurations the coating thickness can change, especially in blind holes or extremely narrow bores or within fritted metal filters like the photo below. The section of the fritted filter shows a band of color. This indicates variation in coating thickness from interior to the exterior of the filter surface. Read on to learn about how color is a general indicator of coating thickness.

3. Base material and surface condition
We coat mostly stainless steel alloys but we can also coat glass, ceramics and more exotic alloys like titanium. The base material can affect the coating rate under certain conditions and add to variation. Also if the base material surface is oxidized, heat stained, etched, buffed, or scratched the coating thickness can vary in those areas.
4. Coating reaction rate
Heat drives most CVD reactions. During processing there's usually a point at which the ideal temperature vs. coating growth rate is achieved. Theoretically, given the right amount of heat, you could build a 1000 nanometer coating in a few seconds or with a minimum of heat build a layer a few days or months! Why not go with lots of heat? Well if you apply lots of heat you're driving the reaction vary fast, much like driving a car fast. And just like trying to stop a fast moving car on a dime, it's hard to stop a fast reaction quickly and precisely.
A fast reaction means you're more likely to overshoot the target layer thickness. So a slow reaction is better? Maybe not. Too slow a reaction, although more likely to yield less thickness variation, will significantly add to processing time and cost . I guess you could specify a coating to the nearest tenth of a nanometer given a super low heat slow reaction. Unfortunately few customer are willing to wait the weeks or months needed to slowly build a coating layer in a low heat reaction.
The ideal chemical vapor deposition strikes a balance between process time, cost and coating thickness variation. SilcoTek's process has optimized cost, time and value for the customer without impacting coating performance. SilcoTek has spent thousands of hours evaluating and optimizing coating performance for each industry application. Optimization involves trade-offs between process and performance. Those trade-offs happen all the time in machine shops, surface finish operations and in virtually all products made today. For paints or other coatings, a user would not notice the difference between say a 500nm coating and an 800nm coating. Our customers notice minor thickness variation in our silicon coatings because the coating color changes with even a slight variation in thickness. What causes the color change?
What does SilcoTek variation look like?
As we discussed last week, we bond silicon to metals, glass, or ceramics through a process called chemical vapor deposition (CVD). This process allows us to build a silicon layer on a surface one Angstrom (0.1 nanometer) at a time. A super thin layer of silicon is pretty clear, in fact you can see through a thin silicon layer. As the silicon thickness increases on say a stainless steel surface, light which travels through the silicon is bent. The light then reflects off the stainless surface and is bent again. All this light bending causes some wavelengths of light to cancel out each other while other wavelengths are reinforced. The canceled (or interference) wavelengths aren't seen by your eye while the reinforced wavelengths hit your cones and are seen as color. Changing the color of a thin silicon surface is easy, a few nanometers change in silicon thickness can have a big impact on the colors you see.
 The best analogy of this effect that I can relate to is oil on water. I'm sure you've noticed that after a rain storm a colorful rainbow oil slick can be found flowing from under that 1966 Mustang that's been parked in your driveway and waiting to be restored for the past 8 years. I know you'll get to it.... Anyway, oil is not colorful, it's either honey colored when new or black when draining from your oil pan. That black oil is becoming a colorful rainbow because of refraction. Light reflects off the top of the oil surface and also travels through the thin oil and is reflected back from the water surface below the oil. Depending on the thickness of the oil, light rays will either be reinforced or interfered with by the oil refraction. When the rays of light reach your eye, the destructive interference wavelengths cancel each other out. The oil thickness variation dictates what colors you see and what color wavelengths get canceled out. So a slight variation in thickness can have a big impact on the color you see.
The best analogy of this effect that I can relate to is oil on water. I'm sure you've noticed that after a rain storm a colorful rainbow oil slick can be found flowing from under that 1966 Mustang that's been parked in your driveway and waiting to be restored for the past 8 years. I know you'll get to it.... Anyway, oil is not colorful, it's either honey colored when new or black when draining from your oil pan. That black oil is becoming a colorful rainbow because of refraction. Light reflects off the top of the oil surface and also travels through the thin oil and is reflected back from the water surface below the oil. Depending on the thickness of the oil, light rays will either be reinforced or interfered with by the oil refraction. When the rays of light reach your eye, the destructive interference wavelengths cancel each other out. The oil thickness variation dictates what colors you see and what color wavelengths get canceled out. So a slight variation in thickness can have a big impact on the color you see.
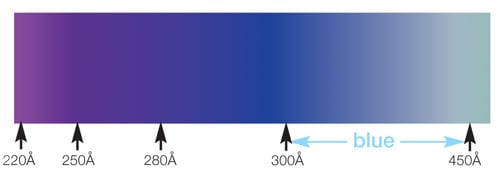
The same holds true with silicon. Small variations in thickness can make a big difference in the color of the coating. So the different colors observed on Silco'd treated parts indicate different layer thicknesses. A blue color corresponds to a 300 to 450 Angstrom (0.3 to 0.45nm) layer while a rainbow color indicates a coating of at least 1200 Angstroms (120 nanometers).
Colors associated with layer thickness are:
Depositions used in our Silcolloy® and SilcoGuard® processes are up to 1µm (1000nm) and have a rainbow to silver/metallic gray appearance. The photos below show colors created by SilcoNert®, a 500nm** coating (left) and Silcolloy®, an 800nm** coating (right). So as you can see, a few nanometers change in thickness will result in a dramatic change in the color of the part.

What tools are used to characterize a surface for optimum coating quality?
Material surface characterization capabilities serve an essential role in supporting SilcoTek® coating science and R&D efforts. The ability to thoroughly evaluate a surface is key to developing new inert barrier coating products, and plays an essential role in assisting customers with scale up and process troubleshooting.
Various surface characterization techniques enable SilcoTek scientists to study materials, analyze surface bonding mechanisms, and develop a deep understanding of surface properties. SilcoTek® production technicians use material characterization tools in their daily QA/QC process to make sure all our inert, corrosion resistant coatings meet our quality standards and satisfy customer needs. Last but not least, it is not uncommon for customers to work with R&D staff to provide characterization help with their specific substrates or coated parts as part of their product development.
Get Our Complete List of
Material Characterization Capabilities
In addition to the in-house characterization capabilities, SilcoTek® also enjoys convenient access to a wide variety of characterization techniques at the Pennsylvania State University (PSU), thanks to our physical proximity to the University’s main campus, and an established academic/industry relationship between PSU and SilcoTek®. SilcoTek’s R&D scientists are certified users of many characterization instruments, like scanning electron microscopes (SEM), located at the Materials Characterization Lab (MCL) at Penn State University. Here are some of the tools we use to support our customer, products and processes.
What Tools Are Needed To Characterize A Surface?
Coating surface characterization starts with a capable and knowledgeable R&D team. Tools to help analyze the surface vary with application and need but here are a few tools we use to understand CVD coatings and surfaces.
X-ray Fluorescence (XRF) Analyzer

In order to avoid process disruptions caused by incompatible substrates, SilcoTek uses a Thermo Scientific X-ray fluorescence (XRF) analyzer, a non-destructive elemental analysis tool, to identify any unfamiliar incoming metal substrates. Why is this important? Because the substrate material affects the coating quality, surface bond, and ultimate product performance. Knowing the base material enables our process technicians to use the most appropriate processing methods to get the best coating results possible.
FTIR
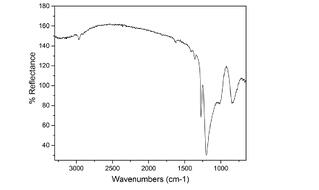
Fourier Transform Infrared Spectroscopy (FTIR) uses broad band infrared radiation as the excitation source to probe molecular structures of various chemical species in gas, liquid or solid state. The FTIR technique is used by SilcoTek’s technicians on a regular basis to perform select QA/QC duties, as well as by SilcoTek’s scientists to assist in their R&D projects. Why is this important? Because FTIR can be used to determine surface quality on a molecular level and can be used as an additional tool in combination with visual inspection criteria to better assess coating quality.
F20 Thin-film Analyzer
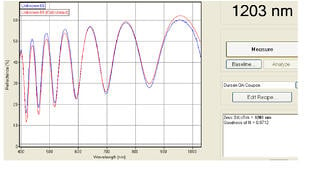
SilcoTek’s F20 thin-film analyzer is a bench top tool that can be configured to measure thin film thicknesses (30Å to 350 mm), optical constants such as refractive index, extinction coefficient and transmittance. It is primarily used by SilcoTek as a quick, accurate and non-destructive way to measure surface thicknesses and to assure consistent coating quality.
Surface contact angle measurement
Contact angle is the angle that a drop of liquid makes to its (usually solid phase) contacting surface. It is measured through the droplet, with the angle formed between the solid surface and the liquid meniscus near the line of contact. The contact angle gives an indication of the wettability of a surface to a liquid (usually water).

SilcoTek scientists rely heavily on the contact angle measurement in their research of hydrophobic and superhydrophobic surface development, where properly designed surface energy can bring out the extreme water-repellent property in a surface or can prevent surface fouling or enhance mold release.
Contact angle measurement us used to benefit SilcoTek’s customers by ensuring the anticipated surface energy (hydrophobicity/hydrophilicity) as required by application is achieved.

Electrochemical Impedance Spectroscopy (EIS)
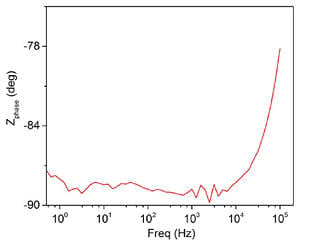
Electrochemical impedance spectroscopy (EIS) is a mostly non-destructive and very useful tool to study and evaluate the performance of protective coatings on metal substrates. The measurement gives information such as resistance, capacitance, double layer capacitance and Faradaic impedance which are related to the performance and failure process of coatings especially during corrosion resistance testing. EIS helps SilcoTek Engineers and Scientists to identify coating pinholes and to develop better, more uniform coatings.
Read More About How
SilcoTek Scientists Improve Coatings
This blog posting highlights a range of materials characterization techniques available to SilcoTek®. It aims to help our customers understand the scope and capability of SilcoTek’s R&D activities, as well as to demonstrate how different tools can be used to facilitate SilcoTek in the development of more innovative solutions, and to help our customers solve application challenges. Easy availability of these tools also allows SilcoTek’s manufacturing team to keep a tight quality control on our inert coating products, thereby providing consistent and reliable products to all our customers.
*Full disclosure, I moved away from the snowy North. While I no longer see snow, I lived in the Northeast for many years and yes I frequently forgot to put my deck furniture in the garage before the first snow.






 The best analogy of this effect that I can relate to is oil on water. I'm sure you've noticed that after a rain storm a colorful rainbow oil slick can be found flowing from under that 1966 Mustang that's been parked in your driveway and waiting to be restored for the past 8 years. I know you'll get to it.... Anyway, oil is not colorful, it's either honey colored when new or black when draining from your oil pan. That black oil is becoming a colorful rainbow because of refraction. Light reflects off the top of the oil surface and also travels through the thin oil and is reflected back from the water surface below the oil. Depending on the thickness of the oil, light rays will either be reinforced or interfered with by the oil refraction. When the rays of light reach your eye, the destructive interference wavelengths cancel each other out. The oil thickness variation dictates what colors you see and what color wavelengths get canceled out. So a slight variation in thickness can have a big impact on the color you see.
The best analogy of this effect that I can relate to is oil on water. I'm sure you've noticed that after a rain storm a colorful rainbow oil slick can be found flowing from under that 1966 Mustang that's been parked in your driveway and waiting to be restored for the past 8 years. I know you'll get to it.... Anyway, oil is not colorful, it's either honey colored when new or black when draining from your oil pan. That black oil is becoming a colorful rainbow because of refraction. Light reflects off the top of the oil surface and also travels through the thin oil and is reflected back from the water surface below the oil. Depending on the thickness of the oil, light rays will either be reinforced or interfered with by the oil refraction. When the rays of light reach your eye, the destructive interference wavelengths cancel each other out. The oil thickness variation dictates what colors you see and what color wavelengths get canceled out. So a slight variation in thickness can have a big impact on the color you see. 







